Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [1]
- Other assay [11]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-29180 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Aspartoacylase Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Description
- Recommended positive controls: mouse brain. Predicted reactivity: Mouse (86%), Rat (86%), Pig (92%), Rhesus Monkey (96%), Bovine (92%). Store product as a concentrated solution. Centrifuge briefly prior to opening the vial.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 µL
- Concentration
- 1 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Mapping the degradation pathway of a disease-linked aspartoacylase variant.
Gersing SK, Wang Y, Grønbæk-Thygesen M, Kampmeyer C, Clausen L, Willemoës M, Andréasson C, Stein A, Lindorff-Larsen K, Hartmann-Petersen R
PLoS genetics 2021 Apr;17(4):e1009539
PLoS genetics 2021 Apr;17(4):e1009539
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western Blot analysis of Aspartoacylase was performed by separating 30 µg of non-transfected (–) and transfected (+) 293T whole cell extracts by 10% SDS-PAGE. Proteins were transferred to a membrane and probed with a Aspartoacylase Polyclonal Antibody (Product # PA5-29180) at a dilution of 1:10000. The HRP-conjugated anti-rabbit IgG antibody was used to detect the primary antibody.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
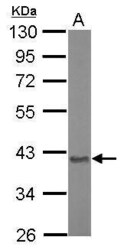
- Experimental details
- Western Blot using Aspartoacylase Polyclonal Antibody (Product # PA5-29180). Sample (20 µg of whole cell lysate). Lane A: Mouse brain. 10% SDS PAGE. Aspartoacylase Polyclonal Antibody (Product # PA5-29180) diluted at 1:10,000.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescent analysis of Aspartoacylase in paraformaldehyde-fixed HeLa cells using an Aspartoacylase polyclonal antibody (Product # PA5-29180) at a 1:500 dilution.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
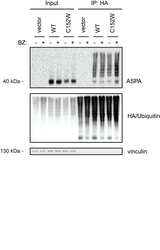
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 1 A growth-based yeast reporter system for protein abundance. (A) Illustration of the reporter system coupling ASPA steady-state levels to yeast growth. Wild-type ASPA and the C152W variant are expressed from the CUP1 promoter with an N-terminal fusion construct consisting of Ura3-HA-GFP. When yeast cells express these constructs, their growth in medium lacking uracil depends on the level of the ASPA fusion protein. (B) The growth of wild-type yeast cells expressing the indicated ASPA fusion protein in liquid medium lacking uracil. The optical density was measured at 600 nm. Error bars represent the standard deviation (n = 4). (C) Wild-type yeast cells expressing the indicated ASPA construct grown on solid medium with or without uracil. (D) Western blot of ASPA protein levels in wild-type yeast expressing the indicated ASPA fusion construct. The ASPA fusion proteins are under the control of the CUP1 promoter, which was either uninduced (-Cu) or induced (+Cu) with copper as shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 2 The ASPA C152W variant is an unstable proteasome target which forms multiple aggregates. (A) Western blot of ASPA protein levels in wild-type yeast expressing the indicated ASPA variant and treated with the translation inhibitor cycloheximide (CHX) for 1 hour (1) or, as a control, with the solvent DMSO (0). Yeast cells were cultured with 0.1 mM CuSO 4 . Blotting for alpha-tubulin was included as a loading control. (B) Growth of wild-type yeast cells expressing the indicated ASPA construct on solid medium without uracil either in absence (no BZ) or in presence (with BZ) of the proteasome inhibitor bortezomib (BZ). (C) Western blot of ASPA protein levels in wild-type yeast expressing the indicated ASPA variant in presence of 0.1 mM CuSO 4 and treated with DMSO (-) or bortezomib (BZ, +) for three hours. Tubulin was included as a loading control. (D) Western blot of soluble (S) and insoluble (P) ASPA protein levels in wild-type yeast expressing the indicated construct in presence of 0.1 mM CuSO 4 . Pma1 and tubulin serve as loading controls for the insoluble and soluble fraction, respectively. (E) Representative fluorescence microscopy images of wild-type yeast cells expressing the indicated ASPA variant. Nuclei were stained with Hoechst. Scale bars are 4 mum. (F) Representative fluorescence microscopy images of wild-type yeast expressing GFP-ASPA C152W and Hsp104-mCherry. ASPA aggregates consistently co-localized with Hsp104, as would be expected for both the JUNQ and the
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 4 Proteasomal degradation of ASPA C152W is mediated by Hsp70, Hsp110, and the E3 ubiquitin-protein ligase Ubr1. (A) Illustration of Hsp70-mediated ASPA degradation in budding yeast. Hsp70 recognizes unfolded and misfolded proteins and interacts with various E3 ubiquitin-protein ligases, potentially resulting in substrate ubiquitination. Subsequently, Hsp70 escorts the ubiquitinated protein to the proteasome where the nucleotide exchange factor (NEF) Hsp110 promotes substrate release, thereby resulting in proteasomal degradation of the substrate protein. (B) Growth of serial-diluted yeast cells of the indicated strains expressing either wild-type ASPA or the C152W variant on solid medium with or without uracil. (C) Western blot showing ASPA protein levels in the indicated strains expressing either wild-type ASPA or C152W in presence of 0.1 mM CuSO 4 . Blotting for alpha-tubulin was used as a loading control. (D) The indicated yeast strains expressing wild-type ASPA or the C152W variant were grown either at 25degC or 30degC prior to protein extraction and Western blotting to examine ASPA protein levels. Tubulin serves as a loading control. (E) The shown strains expressing either wild-type ASPA or the C152W variant were grown at 30degC. Soluble (S) and insoluble (P) protein fractions were separated by centrifugation prior to Western blotting. Pma1 and alpha-tubulin served as loading controls for the insoluble and soluble fractions, respectively. (F) The indicated yeast strai
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 5 Hsp104 dissolves ASPA aggregates to promote protein folding and inclusion body formation. (A) Illustration showing potential outcomes of Hsp104-mediated disaggregation. Hsp104 dissolves protein aggregates, thereby promoting protein folding, sorting of aggregates into inclusion structures, and proteasomal degradation. In addition, the segregase Cdc48 facilitates proteasomal degradation of protein aggregates. (B) Growth of serial-diluted yeast cells of the indicated strains expressing either wild-type ASPA or the C152W variant on medium with or without uracil. (C) Western blot of ASPA protein levels in the indicated strains expressing either wild-type ASPA or C152W in presence of 0.1 mM CuSO 4 . Blotting for alpha-tubulin was used as a loading control. (D) The indicated ASPA constructs were expressed in wild-type or hsp104Delta yeast cells in presence of 0.1 mM CuSO 4 and soluble (S) and insoluble (P) protein was separated by centrifugation prior to Western blotting. Pma1 and alpha-tubulin serve as loading controls for the insoluble and soluble fractions, respectively. (E) Representative fluorescence microscopy images of the indicated strains expressing ASPA C152W. Hoechst dye was used to stain cell nuclei. Scale bars are 4 mum. (F) Cells expressing GFP and containing aggregates were quantified in wild-type (wt, C152W) and hsp104Delta yeast cells expressing the indicated ASPA construct using fluorescence microscopy. The frequencies of cells containing aggregates in separa
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 6 Chaperone-mediated proteasomal degradation of ASPA C152W in human cells. (A) U2OS cells were transiently transfected with the indicated constructs and a GFP expression plasmid to normalize for differences in transfection efficiency. ASPA and GFP protein levels were visualized by Western blotting. Vinculin was used as a loading control. (B) Western blot showing ASPA protein levels in U2OS cells transiently transfected with the indicated constructs prior to treatment with the translation inhibitor cycloheximide (CHX) for 12 hours (+) or the solvent DMSO as a control (-). Vinculin serves as a loading control. (C) U2OS cells were transiently transfected with the indicated constructs prior to treatment with the proteasome inhibitor bortezomib (BZ) for 8 hours (+) or, as a control, the solvent DMSO (-). ASPA protein levels were examined by Western blotting. Blotting for vinculin is used as a loading control. (D) Transiently transfected U2OS cells were treated with the Hsp70 inhibitor YM-01 for 24 hours (+) or the solvent DMSO (-) prior to protein extraction and Western blotting. Vinculin is used as a loading control. (E) U2OS cells were reverse transfected with the shown siRNAs, followed by transient transfection with the constructs shown at the top. Then, ASPA protein levels were examined by Western blotting. Vinculin serves as a loading control. (F) The soluble (S) and insoluble (P) fractions of protein were separated in cell extracts treated as in E prior to Western blotti
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot