Antibody data
- Antibody Data
- Antigen structure
- References [21]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [14]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-9185-82 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD185 (CXCR5) Monoclonal Antibody (MU5UBEE), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The MU5UBEE monoclonal antibody reacts with Human CD185. CD185, which is also known as C-X-C chemokine receptor 5 (CXCR5) and Burkitt lymphoma receptor 1 (BLR1), is a seven transmembrane G protein-coupled receptor originally identified in Burkitt's lymphoma. In peripheral blood, CXCR5 is expressed on B cells, CD4+ T cells (but not Th1 or Th2 cells), as well as on a subpopulation of memory (CD45RO+) T cells. CXCR5+ circulating T cells are in a resting state and migrate to the lymph nodes due to expression of CCR7 and CD62L. In tonsil, CXCR5 is expressed on nearly all CD4+ cells along with CD45RO and such activation markers as CD69 and ICOS. Tonsillar CXCR5+ cells have been shown to induce antibody production when co-cultured with B cells, thus supporting their role in providing B cell help. Furthermore, this chemokine receptor plays a critical role in lymphocyte trafficking, in particular T cell migration into the B cell follicles of germinal centers in response to CXCL13, making CXCR5 an established marker of follicular helper T cells. Applications Reported: This MU5UBEE antibody has been reported for use in flow cytometric analysis. Applications Tested: This MU5UBEE antibody has been tested by flow cytometric analysis of human peripheral blood cells. This can be used at less than or equal to 0.25 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- MU5UBEE
- Vial size
- 100 µg
- Concentration
- 0.5 mg/mL
- Storage
- 4° C
Submitted references Defining Clinical and Immunological Predictors of Poor Immune Responses to COVID-19 mRNA Vaccines in Patients with Primary Antibody Deficiency.
In Situ Characterization of Human Lymphoid Tissue Immune Cells by Multispectral Confocal Imaging and Quantitative Image Analysis; Implications for HIV Reservoir Characterization.
NK-B cell cross talk induces CXCR5 expression on natural killer cells.
Th2 Biased Immunity With Altered B Cell Profiles in Circulation of Patients With Sporotrichosis Caused by Sporothrix globosa.
Non-human Primate Determinants of Natural Killer Cells in Tissues at Steady-State and During Simian Immunodeficiency Virus Infection.
Rapid Transduction and Expansion of Transduced T Cells with Maintenance of Central Memory Populations.
E4BP4-mediated inhibition of T follicular helper cell differentiation is compromised in autoimmune diseases.
Rapid Germinal Center and Antibody Responses in Non-human Primates after a Single Nanoparticle Vaccine Immunization.
S-Geranylgeranyl-L-glutathione is a ligand for human B cell-confinement receptor P2RY8.
Simian Immunodeficiency Virus (SIV)-Specific Chimeric Antigen Receptor-T Cells Engineered to Target B Cell Follicles and Suppress SIV Replication.
Distribution of circulating follicular helper T cells and expression of interleukin-21 and chemokine C-X-C ligand 13 in gastric cancer.
Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches.
Perturbed CD8(+) T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals.
Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection.
Follicular Regulatory CD8 T Cells Impair the Germinal Center Response in SIV and Ex Vivo HIV Infection.
OMIP-025: evaluation of human T- and NK-cell responses including memory and follicular helper phenotype by intracellular cytokine staining.
Cytokine-secreting follicular T cells shape the antibody repertoire.
Systemic immunoregulatory and pathogenic functions of homeostatic chemokine receptors.
A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen.
Sequence variation of a novel heptahelical leucocyte receptor through alternative transcript formation.
Differentiation-specific expression of a novel G protein-coupled receptor from Burkitt's lymphoma.
Shin JJ, Par-Young J, Unlu S, McNamara A, Park HJ, Shin MS, Gee RJ, Doyle H, Afinogenova Y, Zidan E, Kwah J, Russo A, Mamula M, Hsu FI, Catanzaro J, Racke M, Bucala R, Wilen C, Kang I
Journal of clinical immunology 2022 Aug;42(6):1137-1150
Journal of clinical immunology 2022 Aug;42(6):1137-1150
In Situ Characterization of Human Lymphoid Tissue Immune Cells by Multispectral Confocal Imaging and Quantitative Image Analysis; Implications for HIV Reservoir Characterization.
Moysi E, Del Rio Estrada PM, Torres-Ruiz F, Reyes-Terán G, Koup RA, Petrovas C
Frontiers in immunology 2021;12:683396
Frontiers in immunology 2021;12:683396
NK-B cell cross talk induces CXCR5 expression on natural killer cells.
Rascle P, Jacquelin B, Petitdemange C, Contreras V, Planchais C, Lazzerini M, Dereuddre-Bosquet N, Le Grand R, Mouquet H, Huot N, Müller-Trutwin M
iScience 2021 Oct 22;24(10):103109
iScience 2021 Oct 22;24(10):103109
Th2 Biased Immunity With Altered B Cell Profiles in Circulation of Patients With Sporotrichosis Caused by Sporothrix globosa.
Zu J, Yao L, Song Y, Cui Y, Guan M, Chen R, Zhen Y, Li S
Frontiers in immunology 2020;11:570888
Frontiers in immunology 2020;11:570888
Non-human Primate Determinants of Natural Killer Cells in Tissues at Steady-State and During Simian Immunodeficiency Virus Infection.
Huot N, Rascle P, Petitdemange C, Contreras V, Palgen JL, Stahl-Hennig C, Le Grand R, Beignon AS, Jacquelin B, Müller-Trutwin M
Frontiers in immunology 2020;11:2134
Frontiers in immunology 2020;11:2134
Rapid Transduction and Expansion of Transduced T Cells with Maintenance of Central Memory Populations.
Pampusch MS, Haran KP, Hart GT, Rakasz EG, Rendahl AK, Berger EA, Connick E, Skinner PJ
Molecular therapy. Methods & clinical development 2020 Mar 13;16:1-10
Molecular therapy. Methods & clinical development 2020 Mar 13;16:1-10
E4BP4-mediated inhibition of T follicular helper cell differentiation is compromised in autoimmune diseases.
Wang Z, Zhao M, Yin J, Liu L, Hu L, Huang Y, Liu A, Ouyang J, Min X, Rao S, Zhou W, Wu H, Yoshimura A, Lu Q
The Journal of clinical investigation 2020 Jul 1;130(7):3717-3733
The Journal of clinical investigation 2020 Jul 1;130(7):3717-3733
Rapid Germinal Center and Antibody Responses in Non-human Primates after a Single Nanoparticle Vaccine Immunization.
Havenar-Daughton C, Carnathan DG, Boopathy AV, Upadhyay AA, Murrell B, Reiss SM, Enemuo CA, Gebru EH, Choe Y, Dhadvai P, Viviano F, Kaushik K, Bhiman JN, Briney B, Burton DR, Bosinger SE, Schief WR, Irvine DJ, Silvestri G, Crotty S
Cell reports 2019 Nov 12;29(7):1756-1766.e8
Cell reports 2019 Nov 12;29(7):1756-1766.e8
S-Geranylgeranyl-L-glutathione is a ligand for human B cell-confinement receptor P2RY8.
Lu E, Wolfreys FD, Muppidi JR, Xu Y, Cyster JG
Nature 2019 Mar;567(7747):244-248
Nature 2019 Mar;567(7747):244-248
Simian Immunodeficiency Virus (SIV)-Specific Chimeric Antigen Receptor-T Cells Engineered to Target B Cell Follicles and Suppress SIV Replication.
Haran KP, Hajduczki A, Pampusch MS, Mwakalundwa G, Vargas-Inchaustegui DA, Rakasz EG, Connick E, Berger EA, Skinner PJ
Frontiers in immunology 2018;9:492
Frontiers in immunology 2018;9:492
Distribution of circulating follicular helper T cells and expression of interleukin-21 and chemokine C-X-C ligand 13 in gastric cancer.
Meng X, Yu X, Dong Q, Xu X, Li J, Xu Q, Ma J, Zhou C
Oncology letters 2018 Sep;16(3):3917-3922
Oncology letters 2018 Sep;16(3):3917-3922
Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches.
Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Peña A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR
Immunity 2017 Jun 20;46(6):1073-1088.e6
Immunity 2017 Jun 20;46(6):1073-1088.e6
Perturbed CD8(+) T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals.
Tauriainen J, Scharf L, Frederiksen J, Naji A, Ljunggren HG, Sönnerborg A, Lund O, Reyes-Terán G, Hecht FM, Deeks SG, Betts MR, Buggert M, Karlsson AC
Scientific reports 2017 Jan 13;7:40354
Scientific reports 2017 Jan 13;7:40354
Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection.
Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, Williams IR, Skinner PJ, Velu V, Amara RR
Proceedings of the National Academy of Sciences of the United States of America 2017 Feb 21;114(8):1976-1981
Proceedings of the National Academy of Sciences of the United States of America 2017 Feb 21;114(8):1976-1981
Follicular Regulatory CD8 T Cells Impair the Germinal Center Response in SIV and Ex Vivo HIV Infection.
Miles B, Miller SM, Folkvord JM, Levy DN, Rakasz EG, Skinner PJ, Connick E
PLoS pathogens 2016 Oct;12(10):e1005924
PLoS pathogens 2016 Oct;12(10):e1005924
OMIP-025: evaluation of human T- and NK-cell responses including memory and follicular helper phenotype by intracellular cytokine staining.
Moncunill G, Dobaño C, McElrath MJ, De Rosa SC
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2015 Apr;87(4):289-92
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2015 Apr;87(4):289-92
Cytokine-secreting follicular T cells shape the antibody repertoire.
Reinhardt RL, Liang HE, Locksley RM
Nature immunology 2009 Apr;10(4):385-93
Nature immunology 2009 Apr;10(4):385-93
Systemic immunoregulatory and pathogenic functions of homeostatic chemokine receptors.
Müller G, Höpken UE, Stein H, Lipp M
Journal of leukocyte biology 2002 Jul;72(1):1-8
Journal of leukocyte biology 2002 Jul;72(1):1-8
A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen.
Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M
Cell 1996 Dec 13;87(6):1037-47
Cell 1996 Dec 13;87(6):1037-47
Sequence variation of a novel heptahelical leucocyte receptor through alternative transcript formation.
Barella L, Loetscher M, Tobler A, Baggiolini M, Moser B
The Biochemical journal 1995 Aug 1;309 ( Pt 3):773-9
The Biochemical journal 1995 Aug 1;309 ( Pt 3):773-9
Differentiation-specific expression of a novel G protein-coupled receptor from Burkitt's lymphoma.
Dobner T, Wolf I, Emrich T, Lipp M
European journal of immunology 1992 Nov;22(11):2795-9
European journal of immunology 1992 Nov;22(11):2795-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Staining of normal human peripheral blood cells with Anti-Human CD19 APC (Product # 17-0199-42) and 0.125 µg of Mouse IgG2b K Isotype Control Purified (Product # 14-4732-82) (left) or 0.125 µg of Anti-Human CD185 (CXCR5) Purified (right) followed by F (ab')2 Anti-Mouse IgG PE (Product # 12-4010-82).Cells in the lymphocyte gate were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
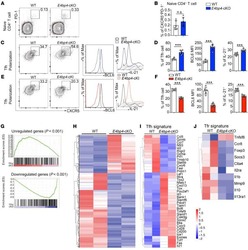
- Example of the staining and gating strategy for PBMC stimulated with Staphylococcal enterotoxin B (SEB). All gates for non-functional markers were defined using fluorescence minus one (FMO) controls whereas gates for functional markers were defined using the unstimulated samples. A : Gating hierarchy to identify NK cells, NKT-like cells, CD4+ and CD8+ T cells, and T FH -like cells. Initial gating is done on FSC-H and FSC-A to discriminate singlets, followed by the exclusion of events collected during a period of time early in collection when fluctuations may occur. In this example, there were no problems of fluctuations and the time gate was minimized to avoid exclusion of any events. Dead cells and monocytes are excluded by an amine reactive dye and the CD14 marker in the same dump channel. Lymphocytes are gated using FSC-A and SSC-A. Subsequent gating discriminates two subsets of NK cells by CD56 and CD3 expression (CD56dimCD3- and CD56hiCD3-) and NKT-like cells (CD56+CD3+). Within the gate of lymphocytes, CD3+ cells are identified, followed by identification of CD4+ and CD8+ T cells. Of note, NKT-like cells are not excluded from classical T cells and therefore are overlapping populations. Finally, T FH -like cells are identified as CXCR5+ CD45RA- CD4+ T cells that have a low expression of CCR7 and are PD-1+. B : Functional markers for CD4+ and CD8+ T cells. A gate is applied for each cytokine, not taking into account the coexpression of other markers. Boolean gates are the
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Extended Immunogen Release Induces Higher nAb Titers Than Conventional Immunization (A-E) Immunogen doses of 100 or 20 mug s.c. immunizations of BG505 SOSIP.664. (A) BG505 nAb titers at week 26 (n = 6 or 12). (B) BG505 SOSIP binding titers at week 26 (n = 6 or 12). (C) Kinetics of BG505 nAb titers. (D and E) GC B cell (D) and GC Tfh cell (E) frequencies after the first, second, and third immunizations. (F-L) Bolus (conventional) versus continuous immunogen delivery of BG505 SOSIP.v5.2 immunogen. (F) Immunization schedule and sampling for continuous antigen delivery using osmotic pumps. (G) BG505 nAb titers in animals immunized by osmotic pump (red) or conventional bolus (Conv, black) ( * p < 0.05; ** p < 0.01; n = 6). (H) Peak BG505 nAb titers after the third immunization (n = 6). (I and J) GC B cell (I) and GC Tfh cell (J) frequencies after the first, second, and third immunizations. (K) Proliferation of GC Tfh cells at week 11. Flow cytometry was gated on CXCR5 hi PD-1 hi GC Tfh cells. (L) Frequency of Ki67 + GC Tfh cells at week 11 (n = 12). All nAb titer and ELISA binding Ab data represent geometric mean titers with geometric SD. All cell-frequency data represent the mean and SD. See also Figure S4 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Unchanged cTfh cells and altered Ig profile in sporotrichosis patients. (A) Statistical graphs in the upper row were for CD4 + CXCR5 + Tfh while the lower row were for CD4 + CXCR5 + PD1 + Tfh. The comparison of cTfh percentages were between HC (n = 24) and whole patients (n = 50), patients with different duration (< 6 mon, n = 24; > 6 mon, n = 26) and clinical types (FF, n = 33; LF, n = 17). (B) Comparison of serum levels of total IgG, IgM, and IgE between patients (n = 46) and HC (n = 24). (C, D) Distribution of IgG subtypes (IgG1, IgG2, and IgG3 and IgG4) in patients (in whole: n = 20; SD: n = 7; LD: n = 13) and HC (n = 19). Error bars represent mean+-SD. * P < 0.05, ** P < 0.01, and NS P >= 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
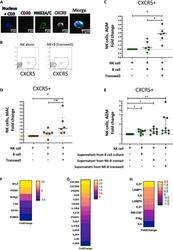
- Figure 1 Temporal and spatial dynamics of CXCR5+ NK cells in peripheral lymph nodes during primary SIVagm infection (A) Gating strategy of CXCR5+ NK cells in pLN. NK cells were gated as CD45 + CD3 - CD20 - NKG2A/C + CXCR5 + cells as previously reported (; , p. 80). A representative image is shown. The example corresponds to pLN cells from an African Green Monkey infected by SIVagm at day 9 post-infection. (B) Kinetic of CXCR5 + NK cell percentages in pLN during SIVagm infection, dpi = days post-infection. Each green circle indicates an individual animal (depending on the time point, n = 3 or 6 AGM); p value = *
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 3 CXCR5+ NK cell induction and gene expression profile (A) Staining of NK cells co-cultured with autologous B cells separated by a transwell membrane illustrating CXCR5 expression on NK cells. The CD3 labeling is used here as a negative control. (B) Dot plot representative of CXCR5+ NK cells from uninfected AGM after 6 days of culture: NK cells cultured alone and NK cells co-cultured with autologous B cells separated by a transwell membrane. (C) The fold change of CXCR5+ NK cell frequencies among total NK cells compared with the frequency of CXCR5+ NK cells in NK cells cultured alone is shown after 6 days of co-culture. B and NK cells were isolated from PBMC of 6 non-infected AGMs (p value = *
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2. Ggg inhibits the migration of P2RY8-expressing cells. (a) P2RY8 ligand bioassay using the indicated concentrations of Ggg, glutathione (GSH), GG-PP, or LTC4 with 50 ng/mL CXCL12 (n=4 biological replicates). (b, c) Transwell migration assays of tonsil cells towards CXCL12 mixed with the indicated concentrations of Ggg. Left plots, representative flow cytometry of CD19 + cells showing the gate for CD38 + IgD - GC B cells (b) or of CD4 + cells showing the gate for PD-1 + , CXCR5 + Tfh cells (c). Right graphs show summary data for indicated cell types. (n=3 tonsils, 2 technical replicates each). (d) Internalization assay using cells expressing OX56 epitope-tagged P2RY8, read by measuring surface OX56 levels (representative flow cytometry histogram, left). Right graphs show summarized data for the indicated receptors (n=6 biological replicates, one-way ANOVA with Bonferroni's multiple comparisons test). Data are pooled from 3 experiments (a,b,c,d). Graphs depict mean with s.d.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 9-Day Transduction and Expansion Protocol Yields Sufficient Cells for Infusion, Which Are Viable and Maintain Co-expression of Two Transduced Genes (A) Total number of cells in culture on days 5 and 9 of the expansion protocol and (B) viability of the transduced cells using PBMCs from seven different animals. Cell number and viability was monitored by trypan blue exclusion counting on the countess cell counter. (C) Representative flow plots showing gating strategy and expression of both the CD4-MBL CAR and CXCR5 in transduced cells on day 9. (D) Expression of CAR, CXCR5, and co-expression of CAR and CXCR5 on days 5 and 9 in cells from seven different animals. The bars represent the mean value and 95% confidence interval.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 Treatment of Macaques with Antiretroviral Drugs Prior to Collection of PBMCs Leads to a Reduction of Transduction Efficiency with Gammaretroviral Vectors PBMCs from six animals using cells collected prior to treatment with anti-retroviral drugs (Pre-ART) or during antiretroviral treatment (ART) were used in the 9-day transduction and expansion protocol. On day 9, cells were evaluated for expression of MBL, representing the CAR, and CXCR5 by flow cytometry. A Wilcoxon matched pairs signed rank test was used to determine significance. Colors denote cells from six individual animals. The bar represents the median value.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2. Longitudinal Sampling of GCs by LN FNA after eOD-GT8 60-mer Immunization (A) Schematic of LN FNA and blood sampling after IM (left) or s.c. (right) immunization. (B) Flow cytometry identification of B GC cell frequencies in the ipsilateral (draining) and contralateral (non-draining) LNs after s.c. and IM immunization. B GC cells are KI67 + BCL6 + . Full gating is Figure S2 . (C) Weekly sampling of B GC cell frequency after one immunization. *p < 0.05, **p < 0.01 (paired t test, one tailed). (D) Flow cytometry identification of GC-T FH cell frequencies in the ipsilateral (draining) and contralateral (non-draining) LNs after s.c. and IM immunization. GC-T FH cells are CXCR5 + PD1 + . (E) Weekly sampling of GC-T FH cell frequency after one immunization. Gated on CD4 + T cells. Each point represents an individual LN FNA sample. n = 8, four LN FNAs per immunization condition at each time point. See also Figures S2 and S3 .
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry