Antibody data
- Antibody Data
- Antigen structure
- References [24]
- Comments [0]
- Validations
- Western blot [1]
- Immunohistochemistry [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 13606-1-AP - Provider product page

- Provider
- Proteintech Group
- Proper citation
- Proteintech Cat#13606-1-AP, RRID:AB_2104403
- Product name
- FBXO22 antibody
- Antibody type
- Polyclonal
- Description
- KD/KO validated FBXO22 antibody (Cat. #13606-1-AP) is a rabbit polyclonal antibody that shows reactivity with human and has been validated for the following applications: IHC, IP, WB,ELISA.
- Reactivity
- Human
- Host
- Rabbit
- Conjugate
- Unconjugated
- Isotype
- IgG
- Vial size
- 20ul, 150ul
Submitted references FBXO22 promotes glioblastoma malignant progression by mediating VHL ubiquitination and degradation.
FBXO22 is a potential therapeutic target for recurrent chondrosarcoma.
Targeting FBXO22 enhances radiosensitivity in non-small cell lung cancer by inhibiting the FOXM1/Rad51 axis.
TANK Binding Kinase 1 Promotes BACH1 Degradation through Both Phosphorylation-Dependent and -Independent Mechanisms without Relying on Heme and FBXO22.
FBXO22 inhibits proliferation and metastasis of cervical cancer cells by mediating ubiquitination-dependent degradation of GAK.
Recruitment of FBXO22 for Targeted Degradation of NSD2.
High dose isoleucine stabilizes nuclear PTEN to suppress the proliferation of lung cancer.
The tRNA-GCN2-FBXO22-axis-mediated mTOR ubiquitination senses amino acid insufficiency.
Fbxo22 inhibits metastasis in triple-negative breast cancer through ubiquitin modification of KDM5A and regulation of H3K4me3 demethylation.
Fbxo22 promotes cervical cancer progression via targeting p57(Kip2) for ubiquitination and degradation.
Biotinylation of an acetylenic tricyclic bis(cyanoenone) lowers its potency as an NRF2 activator while creating a novel activity against BACH1.
BACH1 Expression Is Promoted by Tank Binding Kinase 1 (TBK1) in Pancreatic Cancer Cells to Increase Iron and Reduce the Expression of E-Cadherin.
A small-molecule Skp1 inhibitor elicits cell death by p53-dependent mechanism.
Global identification of phospho-dependent SCF substrates reveals a FBXO22 phosphodegron and an ERK-FBXO22-BAG3 axis in tumorigenesis.
FBXO22 Promotes Growth and Metastasis and Inhibits Autophagy in Epithelial Ovarian Cancers via the MAPK/ERK Pathway.
Pan-Cancer Analyses Reveal Oncogenic Role and Prognostic Value of F-Box Only Protein 22.
FBXO22 Suppresses Metastasis in Human Renal Cell Carcinoma via Inhibiting MMP-9-Mediated Migration and Invasion and VEGF-Mediated Angiogenesis.
FBXO22 mediates polyubiquitination and inactivation of LKB1 to promote lung cancer cell growth.
Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1.
FBXO22 promotes the development of hepatocellular carcinoma by regulating the ubiquitination and degradation of p21.
Phosphorylation of BACH1 switches its function from transcription factor to mitotic chromosome regulator and promotes its interaction with HMMR.
F-Box Protein FBXO22 Mediates Polyubiquitination and Degradation of CD147 to Reverse Cisplatin Resistance of Tumor Cells.
Targeted inhibition of the COP9 signalosome for treatment of cancer.
Quantitative proteome analysis of age-related changes in mouse gastrocnemius muscle using mTRAQ.
Shen Z, Dong T, Yong H, Deng C, Chen C, Chen X, Chen M, Chu S, Zheng J, Li Z, Bai J
Cell death discovery 2024 Mar 23;10(1):151
Cell death discovery 2024 Mar 23;10(1):151
FBXO22 is a potential therapeutic target for recurrent chondrosarcoma.
Xin B, Chen H, Zhu Z, Guan Q, Bai G, Yang C, Zou W, Gao X, Li L, Liu T
Journal of bone oncology 2024 Jun;46:100605
Journal of bone oncology 2024 Jun;46:100605
Targeting FBXO22 enhances radiosensitivity in non-small cell lung cancer by inhibiting the FOXM1/Rad51 axis.
Chen Y, Zhou Y, Feng X, Wu Z, Yang Y, Rao X, Zhou R, Meng R, Dong X, Xu S, Zhang S, Wu G, Jie X
Cell death & disease 2024 Jan 31;15(1):104
Cell death & disease 2024 Jan 31;15(1):104
TANK Binding Kinase 1 Promotes BACH1 Degradation through Both Phosphorylation-Dependent and -Independent Mechanisms without Relying on Heme and FBXO22.
Liu L, Matsumoto M, Watanabe-Matsui M, Nakagawa T, Nagasawa Y, Pang J, Callens BKK, Muto A, Ochiai K, Takekawa H, Alam M, Nishizawa H, Shirouzu M, Shima H, Nakayama K, Igarashi K
International journal of molecular sciences 2024 Apr 9;25(8)
International journal of molecular sciences 2024 Apr 9;25(8)
FBXO22 inhibits proliferation and metastasis of cervical cancer cells by mediating ubiquitination-dependent degradation of GAK.
Li S, Shi L, Wang Y, Zhang L, Chu S, Li M, Bai J, Zhu W
Experimental cell research 2023 Sep 1;430(1):113719
Experimental cell research 2023 Sep 1;430(1):113719
Recruitment of FBXO22 for Targeted Degradation of NSD2.
Nie DY, Tabor JR, Li J, Kutera M, St-Germain J, Hanley RP, Wolf E, Paulakonis E, Kenney TMG, Duan S, Shrestha S, Owens DDG, Pon A, Szewczyk M, Lamberto AJ, Menes M, Li F, Barsyte-Lovejoy D, Brown NG, Barsotti AM, Stamford AW, Collins JL, Wilson DJ, Raught B, Licht JD, James LI, Arrowsmith CH
bioRxiv : the preprint server for biology 2023 Nov 30;
bioRxiv : the preprint server for biology 2023 Nov 30;
High dose isoleucine stabilizes nuclear PTEN to suppress the proliferation of lung cancer.
Wang H, Chen S, Kang W, Ding B, Cui S, Zhou L, Zhang N, Luo H, Wang M, Zhang F, Zhao Z, Guo Z, Wang C, Li L, Wang Z, Chen X, Wang Y
Discover oncology 2023 Feb 23;14(1):25
Discover oncology 2023 Feb 23;14(1):25
The tRNA-GCN2-FBXO22-axis-mediated mTOR ubiquitination senses amino acid insufficiency.
Ge MK, Zhang C, Zhang N, He P, Cai HY, Li S, Wu S, Chu XL, Zhang YX, Ma HM, Xia L, Yang S, Yu JX, Yao SY, Zhou XL, Su B, Chen GQ, Shen SM
Cell metabolism 2023 Dec 5;35(12):2216-2230.e8
Cell metabolism 2023 Dec 5;35(12):2216-2230.e8
Fbxo22 inhibits metastasis in triple-negative breast cancer through ubiquitin modification of KDM5A and regulation of H3K4me3 demethylation.
Li S, He J, Liao X, He Y, Chen R, Chen J, Hu S, Sun J
Cell biology and toxicology 2023 Aug;39(4):1641-1655
Cell biology and toxicology 2023 Aug;39(4):1641-1655
Fbxo22 promotes cervical cancer progression via targeting p57(Kip2) for ubiquitination and degradation.
Lin M, Zhang J, Bouamar H, Wang Z, Sun LZ, Zhu X
Cell death & disease 2022 Sep 20;13(9):805
Cell death & disease 2022 Sep 20;13(9):805
Biotinylation of an acetylenic tricyclic bis(cyanoenone) lowers its potency as an NRF2 activator while creating a novel activity against BACH1.
Moreno R, Casares L, Higgins M, Ali KX, Honda T, Wiel C, Sayin VI, Dinkova-Kostova AT, de la Vega L
Free radical biology & medicine 2022 Oct;191:203-211
Free radical biology & medicine 2022 Oct;191:203-211
BACH1 Expression Is Promoted by Tank Binding Kinase 1 (TBK1) in Pancreatic Cancer Cells to Increase Iron and Reduce the Expression of E-Cadherin.
Liu L, Matsumoto M, Matsui-Watanabe M, Ochiai K, Callens BKK, Nguyen LC, Kozuki Y, Tanaka M, Nishizawa H, Igarashi K
Antioxidants (Basel, Switzerland) 2022 Jul 27;11(8)
Antioxidants (Basel, Switzerland) 2022 Jul 27;11(8)
A small-molecule Skp1 inhibitor elicits cell death by p53-dependent mechanism.
Hussain M, Lu Y, Tariq M, Jiang H, Shu Y, Luo S, Zhu Q, Zhang J, Liu J
iScience 2022 Jul 15;25(7):104591
iScience 2022 Jul 15;25(7):104591
Global identification of phospho-dependent SCF substrates reveals a FBXO22 phosphodegron and an ERK-FBXO22-BAG3 axis in tumorigenesis.
Liu P, Cong X, Liao S, Jia X, Wang X, Dai W, Zhai L, Zhao L, Ji J, Ni D, Liu Z, Chen Y, Pan L, Liu W, Zhang J, Huang M, Liu B, Tan M
Cell death and differentiation 2022 Jan;29(1):1-13
Cell death and differentiation 2022 Jan;29(1):1-13
FBXO22 Promotes Growth and Metastasis and Inhibits Autophagy in Epithelial Ovarian Cancers via the MAPK/ERK Pathway.
Li M, Zhao X, Yong H, Shang B, Lou W, Wang Y, Bai J
Frontiers in pharmacology 2021;12:778698
Frontiers in pharmacology 2021;12:778698
Pan-Cancer Analyses Reveal Oncogenic Role and Prognostic Value of F-Box Only Protein 22.
Chen S, Ma S, Yan J, Wang H, Ding B, Guo Z, Ma Y, Chen X, Wang Y
Frontiers in oncology 2021;11:790912
Frontiers in oncology 2021;11:790912
FBXO22 Suppresses Metastasis in Human Renal Cell Carcinoma via Inhibiting MMP-9-Mediated Migration and Invasion and VEGF-Mediated Angiogenesis.
Guo F, Liu J, Han X, Zhang X, Lin T, Wang Y, Bai J, Han J
International journal of biological sciences 2019;15(3):647-656
International journal of biological sciences 2019;15(3):647-656
FBXO22 mediates polyubiquitination and inactivation of LKB1 to promote lung cancer cell growth.
Zhu XN, He P, Zhang L, Yang S, Zhang HL, Zhu D, Liu MD, Yu Y
Cell death & disease 2019 Jun 19;10(7):486
Cell death & disease 2019 Jun 19;10(7):486
Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1.
Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, Pass HI, Bhutkar AJ, Tsirigos A, Ueberheide B, Sayin VI, Papagiannakopoulos T, Pagano M
Cell 2019 Jul 11;178(2):316-329.e18
Cell 2019 Jul 11;178(2):316-329.e18
FBXO22 promotes the development of hepatocellular carcinoma by regulating the ubiquitination and degradation of p21.
Zhang L, Chen J, Ning D, Liu Q, Wang C, Zhang Z, Chu L, Yu C, Liang HF, Zhang B, Chen X
Journal of experimental & clinical cancer research : CR 2019 Feb 26;38(1):101
Journal of experimental & clinical cancer research : CR 2019 Feb 26;38(1):101
Phosphorylation of BACH1 switches its function from transcription factor to mitotic chromosome regulator and promotes its interaction with HMMR.
Li J, Shima H, Nishizawa H, Ikeda M, Brydun A, Matsumoto M, Kato H, Saiki Y, Liu L, Watanabe-Matsui M, Iemura K, Tanaka K, Shiraki T, Igarashi K
The Biochemical journal 2018 Mar 15;475(5):981-1002
The Biochemical journal 2018 Mar 15;475(5):981-1002
F-Box Protein FBXO22 Mediates Polyubiquitination and Degradation of CD147 to Reverse Cisplatin Resistance of Tumor Cells.
Wu B, Liu ZY, Cui J, Yang XM, Jing L, Zhou Y, Chen ZN, Jiang JL
International journal of molecular sciences 2017 Jan 20;18(1)
International journal of molecular sciences 2017 Jan 20;18(1)
Targeted inhibition of the COP9 signalosome for treatment of cancer.
Schlierf A, Altmann E, Quancard J, Jefferson AB, Assenberg R, Renatus M, Jones M, Hassiepen U, Schaefer M, Kiffe M, Weiss A, Wiesmann C, Sedrani R, Eder J, Martoglio B
Nature communications 2016 Oct 24;7:13166
Nature communications 2016 Oct 24;7:13166
Quantitative proteome analysis of age-related changes in mouse gastrocnemius muscle using mTRAQ.
Hwang CY, Kim K, Choi JY, Bahn YJ, Lee SM, Kim YK, Lee C, Kwon KS
Proteomics 2014 Jan;14(1):121-32
Proteomics 2014 Jan;14(1):121-32
No comments: Submit comment
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image

- Experimental details
- HeLa cells were subjected to SDS PAGE followed by western blot with 13606-1-AP(FBXO22 antibody) at dilution of 1:500
- Sample type
- cell line
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image
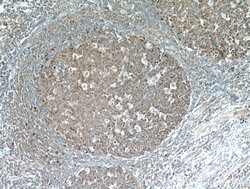
- Experimental details
- Immunohistochemical of paraffin-embedded human tonsil using 13606-1-AP(FBXO22 antibody) at dilution of 1:50 (under 10x lens)
- Sample type
- tissue
- Submitted by
- Proteintech Group (provider)
- Main image
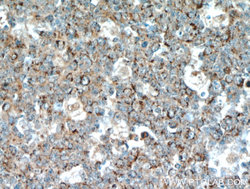
- Experimental details
- The FBXO22 antibody from Proteintech is a rabbit polyclonal antibody to a recombinant protein of human FBXO22. This antibody recognizes human,mouse,rat antigen. The FBXO22 antibody has been validated for the following applications: ELISA, WB, IHC analysis.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA