Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Western blot [1]
- Other assay [3]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-28457 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- MID2 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- Recommended positive controls: MID2 tansfected 293T cell. Predicted reactivity: Mouse (100%), Rat (100%). Store product as a concentrated solution. Centrifuge briefly prior to opening the vial.
- Reactivity
- Human
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 µL
- Concentration
- 1 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references The E3 ligase TRIM1 ubiquitinates LRRK2 and controls its localization, degradation, and toxicity.
Stormo AED, Shavarebi F, FitzGibbon M, Earley EM, Ahrendt H, Lum LS, Verschueren E, Swaney DL, Skibinski G, Ravisankar A, van Haren J, Davis EJ, Johnson JR, Von Dollen J, Balen C, Porath J, Crosio C, Mirescu C, Iaccarino C, Dauer WT, Nichols RJ, Wittmann T, Cox TC, Finkbeiner S, Krogan NJ, Oakes SA, Hiniker A
The Journal of cell biology 2022 Apr 4;221(4)
The Journal of cell biology 2022 Apr 4;221(4)
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
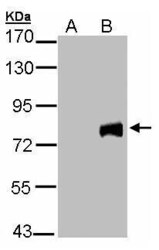
- Experimental details
- Western Blot analysis of MID2 expression in transfected 293T cell line by MID2 polyclonal antibody. Lane A: Non-transfected lysate. B: MID2 transfected lysate. 12% SDS PAGE. MID2 Polyclonal Antibody (Product # PA5-28457) diluted at 1:5,000.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
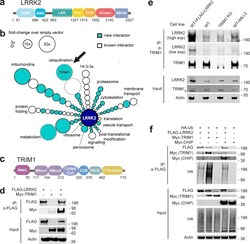
- Experimental details
- Figure 1. TRIM1 is a new LRRK2 E3 ubiquitin ligase. (a) Diagram of LRRK2 protein domains (ARM, armadillo repeat; ANK, ankyrin repeat; LRR, leucine-rich repeat; ROC, ras of complex proteins; COR, C-terminal of ROC domain). (b) Schematic of LRRK2 interactome in HEK-293T cells. LRRK2 interacting partners are classified radially according to function (aqua, new LRRK2 interacting partners; white, previously identified LRRK2 partners; size of circle indicates fold-change over empty vector control; circles without solid black outline had no peptides present in empty vector control; arrow indicates TRIM1). FLAG-LRRK2 was immunoprecipitated and interacting partners were identified and quantified by MS. Data represent at least four total independent replicates from two experiments and are additionally shown in Table S1 . (c) Diagram of TRIM1 protein domains (FNIII, fibronectin III domain). (d) Coimmunoprecipitation of myc-TRIM1 with FLAG-LRRK2 in HEK-293T cells. (e) Coimmunoprecipitation of endogenous LRRK2 with TRIM1 in WT HEK-293T and HEK-293T TRIM1 CRISPR KO line. From left to right: WT HEK-293T cells transfected with exogenous FLAG-LRRK2 (positive control), WT HEK-293T cells, TRIM1 KO HEK-293T cells, and WT HEK-293T cells treated with 500 nM MLi-2 for 5 h. Low exp, short exposure of membrane; high exp, longer exposure of membrane. (f) Coimmunoprecipitation and ubiquitination of FLAG-LRRK2 with myc-TRIM1 or myc-CHIP in the presence of HA-ubiquitin in HEK-293T cells. Source data are
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
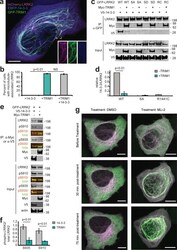
- Experimental details
- Figure 6. TRIM1 competes with 14-3-3 to bind LRRK2's regulatory loop and recruit LRRK2 to microtubules. (a) Live-cell confocal microscopy of mCherry-LRRK2 in the presence of EBFP2-14-3-3 and GFP-TRIM1. Scale bar = 10 muM. (b) Quantification of H1299 cells with microtubule-associated LRRK2 when coexpressed with indicated proteins. 100 cells were evaluated in each experiment; bars indicate mean +- SD (two independent experiments). (c) Coimmunoprecipitation of GFP-LRRK2 WT, Ser910Ala Ser935Ala (SA), Ser910Asp Ser935Asp (SD), or R1441C (RC) with V5-14-3-3 theta in the presence and absence of myc-TRIM1 in HEK-293T cells. (d) Quantification of panel c showing mean values from three independent experiments, with error bars showing +- SD. Significance determined by Mann-Whitney U test. (e) Coimmunoprecipitation of GFP-LRRK2 with either V5-14-3-3 theta or myc-TRIM1 in HEK-293T cells. Overlaid immunoblots in color show relative ratio of phospho- to total-LRRK2 (total LRRK2 in green, antibody is NeuroMab clone N241A/34; phospho-LRRK2 is in red, antibodies are phospho-Ser910 [Abcam, UDD 1 15(3)] and phospho-Ser935 [UDD 2 10(12); Abcam]). (f) Quantification of e with mean +- SEM from three independent experiments. (g) Live-cell confocal microscopy of GFP-LRRK2 in the presence of mCherry-TRIM1 after treatment with LRRK2 kinase inhibitor MLi-2 (200 nM) or vehicle. Rare cells with low levels of colocalization before treatment were followed. LRRK2 is shown in green and TRIM1 in purple. Images
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
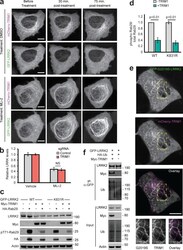
- Experimental details
- Figure S5. Additional c haracterization of effect of TRIM1 on LRRK2 localization and function. (a) Live-cell confocal microscopy of GFP-LRRK2 in the presence of mCherry-TRIM1 after treatment with LRRK2 kinase inhibitor MLi-2 (200 nM) or vehicle, showing the individual mCherry and GFP channels from the time course in Fig. 6 g . Rare cells with low levels of colocalization before treatment were followed over time. Scale bar = 10 muM. (b) Quantification of GFP-LRRK2 fluorescence in flow cytometric assay with TRIM1 knocked down (red bar) compared with cells with nontargeting sgRNA (gray bar). Cells were dox-induced for 24 h, dox was removed, and MLi-2 (100 nM) or vehicle was added for another 24 h before cells were assayed. Bars show median green fluorescence intensity, with error bars showing twice the SEM. (c) Immunoblot of Rab29 phosphorylation in the presence and absence of TRIM1 for WT LRRK2 and LRRK2 K831R. (d) Quantification of Rab29 phosphorylation in panel c. (e) Live-cell confocal microscopy of GFP-LRRK2 G2019S and mCherry-TRIM1 transiently transfected into PC-12 cells (scale bar = 5 muM). From top to bottom: GFP-LRRK2 G2019S, mCherry-TRIM1, merged image, and higher magnification of area in yellow boxes. (f) Immunoprecipitation and ubiquitination of GFP-LRRK2 with myc-TRIM1 in the presence of HA-ubiquitin in PC12 cells. The immunoblot membrane was physically cut between LRRK2- and myc-blotted portions, with both sections additionally probed with an anti-ubiquitin primar
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot