Antibody data
- Antibody Data
- Antigen structure
- References [17]
- Comments [0]
- Validations
- Immunohistochemistry [1]
- Other assay [8]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-9771-80 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Collagen X Monoclonal Antibody (X53), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The monoclonal antibody X53 recognizes human, mouse, porcine, guinea pig, and rat Collagen Type X. X53 does not cross-react with collagen types I, II, III, IV, or VI. Collagen Type X is a homotrimeric short chain collagen. Collagen Type X has limited expression in normal adult tissues, primarily being expressed during bone growth and development. Expression is localized to the peri- and extracellular matrix of hypertrophic chondrocytes during endochondral ossification and also plays a role in cartilage mineralization. Collagen Type X is elevated in many tumor types with expression being localized in the vasculature. Mutations to the gene COL10A1, which encodes human Collagen Type X, is associated with Schmid type metaphyseal chondrodysplasia. Applications Reported: This X53 antibody has been reported for use in microscopy, immunohistochemical staining, ELISA, and immunocytochemistry. Applications Tested: This X53 antibody has been tested by immunohistochemistry on formalin-fixed paraffin embedded human tissue using low pH antigen retrieval and can be used at less than or equal to 10 µg/mL. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human, Mouse, Rat, Canine, Guinea Pig, Porcine
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- X53
- Vial size
- 25 µg
- Concentration
- 0.5 mg/mL
- Storage
- 4° C
Submitted references Spatiotemporal distribution of thrombospondin-4 and -5 in cartilage during endochondral bone formation and repair.
The Effects of Fluvastatin on Indian Hedgehog Pathway in Endochondral Ossification.
Angiogenic Potential of Tissue Engineered Cartilage From Human Mesenchymal Stem Cells Is Modulated by Indian Hedgehog and Serpin E1.
Control of skeletal morphogenesis by the Hippo-YAP/TAZ pathway.
Perlecan Knockdown Significantly Alters Extracellular Matrix Composition and Organization During Cartilage Development.
Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice.
Lipid phosphatase SHIP-1 regulates chondrocyte hypertrophy and skeletal development.
Promoting Effect of Basic Fibroblast Growth Factor in Synovial Mesenchymal Stem Cell-Based Cartilage Regeneration.
Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head.
Oral administration of EP4-selective agonist KAG-308 suppresses mouse knee osteoarthritis development through reduction of chondrocyte hypertrophy and TNF secretion.
Identification of stromal ColXα1 and tumor-infiltrating lymphocytes as putative predictive markers of neoadjuvant therapy in estrogen receptor-positive/HER2-positive breast cancer.
A Hydrogel Model Incorporating 3D-Plotted Hydroxyapatite for Osteochondral Tissue Engineering.
COL10A1 expression is elevated in diverse solid tumor types and is associated with tumor vasculature.
Characterization of human type X procollagen and its NC-1 domain expressed as recombinant proteins in HEK293 cells.
Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies.
The gonadotrophin-releasing hormone receptor of alpha T3-1 pituitary cells regulates cellular levels of both of the phosphoinositidase C-linked G proteins, Gq alpha and G11 alpha, equally.
Additional mutations of type X collagen confirm COL10A1 as the Schmid metaphyseal chondrodysplasia locus.
Andrés Sastre E, Maly K, Zhu M, Witte-Bouma J, Trompet D, Böhm AM, Brachvogel B, van Nieuwenhoven CA, Maes C, van Osch GJVM, Zaucke F, Farrell E
Bone 2021 Sep;150:115999
Bone 2021 Sep;150:115999
The Effects of Fluvastatin on Indian Hedgehog Pathway in Endochondral Ossification.
Ishikawa M, Ishii T, Morikawa T, Iijima Y, Sueishi K
Cartilage 2021 Dec;13(2_suppl):304S-314S
Cartilage 2021 Dec;13(2_suppl):304S-314S
Angiogenic Potential of Tissue Engineered Cartilage From Human Mesenchymal Stem Cells Is Modulated by Indian Hedgehog and Serpin E1.
Nossin Y, Farrell E, Koevoet WJLM, Somoza RA, Caplan AI, Brachvogel B, van Osch GJVM
Frontiers in bioengineering and biotechnology 2020;8:327
Frontiers in bioengineering and biotechnology 2020;8:327
Control of skeletal morphogenesis by the Hippo-YAP/TAZ pathway.
Vanyai HK, Prin F, Guillermin O, Marzook B, Boeing S, Howson A, Saunders RE, Snoeks T, Howell M, Mohun TJ, Thompson B
Development (Cambridge, England) 2020 Nov 12;147(21)
Development (Cambridge, England) 2020 Nov 12;147(21)
Perlecan Knockdown Significantly Alters Extracellular Matrix Composition and Organization During Cartilage Development.
Ocken AR, Ku MM, Kinzer-Ursem TL, Calve S
Molecular & cellular proteomics : MCP 2020 Jul;19(7):1220-1235
Molecular & cellular proteomics : MCP 2020 Jul;19(7):1220-1235
Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice.
Niedermair T, Straub RH, Brochhausen C, Grässel S
International journal of molecular sciences 2020 Jan 8;21(2)
International journal of molecular sciences 2020 Jan 8;21(2)
Lipid phosphatase SHIP-1 regulates chondrocyte hypertrophy and skeletal development.
So EY, Sun C, Wu KQ, Driesman A, Leggett S, Isaac M, Spangler T, Dubielecka-Szczerba PM, Reginato AM, Liang OD
Journal of cellular physiology 2020 Feb;235(2):1425-1437
Journal of cellular physiology 2020 Feb;235(2):1425-1437
Promoting Effect of Basic Fibroblast Growth Factor in Synovial Mesenchymal Stem Cell-Based Cartilage Regeneration.
Okamura G, Ebina K, Hirao M, Chijimatsu R, Yonetani Y, Etani Y, Miyama A, Takami K, Goshima A, Yoshikawa H, Ishimoto T, Nakano T, Hamada M, Kanamoto T, Nakata K
International journal of molecular sciences 2020 Dec 30;22(1)
International journal of molecular sciences 2020 Dec 30;22(1)
Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head.
Herrmann M, Hildebrand M, Menzel U, Fahy N, Alini M, Lang S, Benneker L, Verrier S, Stoddart MJ, Bara JJ
International journal of molecular sciences 2019 Jul 14;20(14)
International journal of molecular sciences 2019 Jul 14;20(14)
Oral administration of EP4-selective agonist KAG-308 suppresses mouse knee osteoarthritis development through reduction of chondrocyte hypertrophy and TNF secretion.
Murahashi Y, Yano F, Chijimatsu R, Nakamoto H, Maenohara Y, Amakawa M, Miyake Y, Yamanaka H, Iba K, Yamashita T, Tanaka S, Saito T
Scientific reports 2019 Dec 30;9(1):20329
Scientific reports 2019 Dec 30;9(1):20329
Identification of stromal ColXα1 and tumor-infiltrating lymphocytes as putative predictive markers of neoadjuvant therapy in estrogen receptor-positive/HER2-positive breast cancer.
Brodsky AS, Xiong J, Yang D, Schorl C, Fenton MA, Graves TA, Sikov WM, Resnick MB, Wang Y
BMC cancer 2016 Apr 18;16:274
BMC cancer 2016 Apr 18;16:274
A Hydrogel Model Incorporating 3D-Plotted Hydroxyapatite for Osteochondral Tissue Engineering.
Bartnikowski M, Akkineni AR, Gelinsky M, Woodruff MA, Klein TJ
Materials (Basel, Switzerland) 2016 Apr 14;9(4)
Materials (Basel, Switzerland) 2016 Apr 14;9(4)
COL10A1 expression is elevated in diverse solid tumor types and is associated with tumor vasculature.
Chapman KB, Prendes MJ, Sternberg H, Kidd JL, Funk WD, Wagner J, West MD
Future oncology (London, England) 2012 Aug;8(8):1031-40
Future oncology (London, England) 2012 Aug;8(8):1031-40
Characterization of human type X procollagen and its NC-1 domain expressed as recombinant proteins in HEK293 cells.
Frischholz S, Beier F, Girkontaite I, Wagner K, Pöschl E, Turnay J, Mayer U, von der Mark K
The Journal of biological chemistry 1998 Feb 20;273(8):4547-55
The Journal of biological chemistry 1998 Feb 20;273(8):4547-55
Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies.
Girkontaite I, Frischholz S, Lammi P, Wagner K, Swoboda B, Aigner T, Von der Mark K
Matrix biology : journal of the International Society for Matrix Biology 1996 Sep;15(4):231-8
Matrix biology : journal of the International Society for Matrix Biology 1996 Sep;15(4):231-8
The gonadotrophin-releasing hormone receptor of alpha T3-1 pituitary cells regulates cellular levels of both of the phosphoinositidase C-linked G proteins, Gq alpha and G11 alpha, equally.
Shah BH, Milligan G
Molecular pharmacology 1994 Jul;46(1):1-7
Molecular pharmacology 1994 Jul;46(1):1-7
Additional mutations of type X collagen confirm COL10A1 as the Schmid metaphyseal chondrodysplasia locus.
McIntosh I, Abbott MH, Warman ML, Olsen BR, Francomano CA
Human molecular genetics 1994 Feb;3(2):303-7
Human molecular genetics 1994 Feb;3(2):303-7
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
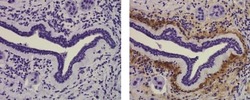
- Immunohistochemistry of formalin-fixed paraffin embedded human infiltrating ductal carcinoma tissue using 10 µg/mL of Mouse IgG1 K Isotype Control Purified (left) or 10 µg/mL of Anti-Collagen Type X Purified (right) followed by Anti-Mouse IgG Biotin, Streptavidin HRP, and DAB visualization.Nuclei are counterstained with hematoxylin.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
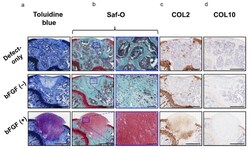
- Figure 7 In vivo osteochondral repair in a mouse model: evaluation of chondrogenic repair ( a ) Histology of the tissue stained with toluidine blue (bar = 500 um). ( b ) Histology of the tissue stained with Saf-O (left columns; bar = 500 um) and the corresponding high-magnification images (right columns; bar = 100 um). ( c ) Immunohistochemical analysis of repair cartilage stained for COL2 (bar = 500 um). ( d ) Immunohistochemical analysis of repair tissue stained for COL10 (bar = 500 um). bFGF, basic fibroblast growth factor; Saf-O, Safranin-O; COL2, collagen type II; COL10, collagen type X.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
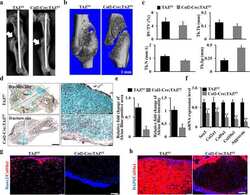
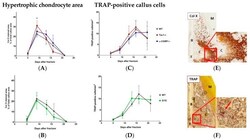
- Figure 5 Hypertrophic cartilage area and number of TRAP-positive callus cells . ( A , B ) Number/area of hypertrophic chondrocytes, visualized by collagen X staining, in Tac1-/-, alpha-CGRP-/- ( A ), SYX ( B ), and WT mice ( A , B ) at the time points 5, 9, 13, 16, and 21 days after fracture. Values were calculated as a percentage of total callus area and are presented as mean +- SD. * in black indicates differences between neurotransmitter deficient mice and WT animals. ( C , D ) Number of TRAP-positive cells in the callus tissue of Tac1-/-, alpha-CGRP-/- ( C ), SYX ( D ), and WT mice ( C , D ) at the time points 5, 9, 13, 16, and 21 days after fracture. The number of cells was calculated in relation to the total area of callus tissue as number/mm 2 . ( E ) Representative image of collagen X stained hypertrophic callus area of an alpha-CGRP-/- mouse 13 days after fracture. ( F ) Representative image of TRAP-positive callus cells 16 days after fracture in the callus tissue of a WT mouse. Overview images scanned with 20x magnification (TissueFAXSi plus). Red boxes demonstrate the enlarged view. Red arrow points to stained cells. Col X = collagen X; B = bone, C = Callus tissue, F = Fracture site, M = Muscle. * = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- FIGURE 1 Chondrogenic differentiation of bone marrow derived mesenchymal stem cells (BMSC). (A) Histological and Immunohistological staining of day 21 chondrogenically differentiated BMSC-pellets of the donors for Collagen type II, Thionine and Collagen type X. (B) qPCR Gene-expression analysis of chondrocyte and hypertrophy marker genes in day 21 chondrogenically differentiated BMSCs. Raw average Ct value for COL2A1 = 20.54; COL10A1 = 20.99; ALPL = 28.19.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 Effects of KAG-308 on chondrocyte hypertrophy in vitro . ( a ) mRNA levels of Col10a1 , Runx2 , Mef2c , and Col2a1 in pellet culture of mouse articular chondrocytes treated with indicated concentration of KAG-308 for 2 wks. Symbols represent individual pellets; long and short bars show the mean and SD, respectively. * P < 0.05, ** P < 0.005, *** P < 0.0005 vs vehicle by ANOVA followed by Dunnett's post hoc test. ( b ) Safranin O staining and Col10a1 immunostaining of cultured pellets of mouse articular chondrocytes treated with KAG-308 for 2 wks. Inset boxes in safranin-O staining indicate enlarged images. Scale bar, 200 um. ( c ) Immunoblotting of Hdac4 in nuclear and cytosolic fractions from mouse articular chondrocytes in a time course with 10 nM KAG-308 treatment. Right graphs indicate quantitative densitometry analysis of the immunoblots (n = 5). Hdac4 values were normalized to Pcna (nuclear) or Actin (cytosol). Long and short bars show the mean and SD, respectively. * P < 0.05, ** P < 0.005 vs 0 hour by ANOVA followed by Dunnett's post hoc test. ( d ) Immunocytochemistry of Hdac4 in mouse articular chondrocytes after 4-d-culture in BMP-2-containing medium with vehicle or 10 nM KAG-308. Right graph shows the percent of cells with nuclear-localized Hdac4. Symbols represent independent slides; long and short bars show the mean and SD, respectively. * P < 0.0005 by Welch's t test. Scale bar, 20 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 Accelerated chondrogenesis in SHIP-1 KO PalphaS MSCs. PalphaS MSC pellets differentiated in vitro for 21 days using the Mouse StemXVivo Chondrogenic Base Media and Supplement were fixed, paraffin embedded, sectioned and IHC-stained. (a and b) Sections from WT (a) and SHIP-1 KO (b) PalphaS MSC pellets were stained with an antibody against the early chondrogenic lineage marker Col II. Bound antibody was visualized in bright red with a NorthernLights 557-conjugated anti-sheep secondary antibody. (c and d) Sections from WT (c) and SHIP-1 KO (d) PalphaS MSC pellets were stained with a bright red eFluor570-conjugated antibody against the hypertrophic chondrocyte lineage marker Col X. (e and f) Sequential sections from the same WT cell pellet as in (c) and from the same SHIP-1 KO cell pellet as in (d) were subjected to H&E staining. Magnification: x200 (a-d); x400 (e and f). Scale bar = 50 mum. (g) Left panel, the difference in cell sizes between WT and SHIP-1 KO PalphaS MSCs after chondrogenic induction was quantified by using the NIH ImageJ program and indicated as ImageJ Area Units. Middle and right panels, gene expression levels of Col2a1 and Col10a1 were evaluated by quantitative real-time PCR. GAPDH expression was assessed as an internal reference for quantification. Gene expression levels were expressed as relative fold increase over the internal control. Student's t test was performed and p < .05 is considered significant. Col II, collagen type II; Col X, collagen t
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
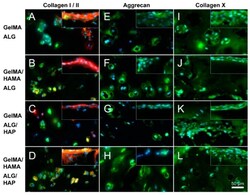
- Experimental details
- Figure 8 Immunofluorescence images illustrating deep and surface sections. Staining of collagen I (red), collagen II (green), aggrecan (green) and collagen X (green) was performed on constructs from all groups on day 28 (as indicated; DAPI nuclei staining in light blue); ( A , E , I ): GelMA-ALG; ( B , F , J ): GelMA/HAMA-ALG; ( C , G , K ): GelMA-ALG/HAP and ( D , H , L ): GelMA/HAMA-ALG/HAP. Images were taken from the deep hydrogel zone near to the ALG or ALG/HAP scaffold, with surface images inset; scale bar 50 um.
 Explore
Explore Validate
Validate Learn
Learn ELISA
ELISA Immunohistochemistry
Immunohistochemistry