Antibody data
- Antibody Data
- Antigen structure
- References [106]
- Comments [0]
- Validations
- Western blot [5]
- Immunocytochemistry [1]
- Other assay [40]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-5825-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- T-bet Monoclonal Antibody (eBio4B10 (4B10)), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The eBio4B10 monoclonal antibody reacts with mouse and human T-bet. T-bet is a Th1-specific T-box transcription factor critical to the development of the Th1 CD4+ T cell lineage. This is known based on the observations that T-Bet deficient mice have impaired Th1 cell development, and that ectopic expression of T-Bet results in development skewed to the Th1 lineage. T-Bet expression is induced by the Th1 cytokine IFN gamma, and T-Bet also regulates the expression of IFN gamma, likely, at least in part, through the modification of DNA accessibility and histone remodeling. In addition to IFN gamma, T-Bet is also known to regulate the expression of IL-12R beta and IL-2. Moreover, T-Bet plays a role in class-switch recombination in B-cells. Applications Reported: This eBio4B10 antibody has been reported for use in immunohistochemical staining of formalin-fixed paraffin embedded tissue sections (with citrate antigen retrieval), immunoprecipitation, and immunoblotting (WB). Applications Tested: This eBio4B10 antibody has been tested by western blot analysis of Th1-polarized mouse CD4+ T cells and can be used at 2 µg/mL as a starting dilution for western blotting. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human, Mouse
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- eBio4B10 (4B10)
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4° C
Submitted references Graded RhoA GTPase Expression in Treg Cells Distinguishes Tumor Immunity From Autoimmunity.
Immune cell phenotypes associated with disease severity and long-term neutralizing antibody titers after natural dengue virus infection.
Role of nuclear localization in the regulation and function of T-bet and Eomes in exhausted CD8 T cells.
Heterologous arenavirus vector prime-boost overrules self-tolerance for efficient tumor-specific CD8 T cell attack.
STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer.
Culture, expansion, and flow-cytometry-based functional analysis of pteropid bat MR1-restricted unconventional T cells.
Francisella tularensis induces Th1 like MAIT cells conferring protection against systemic and local infection.
Splicing factor SRSF1 limits IFN-γ production via RhoH and ameliorates experimental nephritis.
Targeting Phosphatidylserine Enhances the Anti-tumor Response to Tumor-Directed Radiation Therapy in a Preclinical Model of Melanoma.
ADAM12 is a costimulatory molecule that determines Th1 cell fate and mediates tissue inflammation.
Imbalance between T helper 1 and regulatory T cells plays a detrimental role in experimental Parkinson's disease in mice.
Th2 Biased Immunity With Altered B Cell Profiles in Circulation of Patients With Sporotrichosis Caused by Sporothrix globosa.
Mir-124 Attenuates STAT3-Mediated TH17 Differentiation in Colitis-Driven Colon Cancer.
MiR-1165-3p Suppresses Th2 Differentiation via Targeting IL-13 and PPM1A in a Mouse Model of Allergic Airway Inflammation.
Programming Multifaceted Pulmonary T Cell Immunity by Combination Adjuvants.
CD19-CAR-T Cells Bearing a KIR/PD-1-Based Inhibitory CAR Eradicate CD19(+)HLA-C1(-) Malignant B Cells While Sparing CD19(+)HLA-C1(+) Healthy B Cells.
Intestinal vitamin D receptor knockout protects from oxazolone-induced colitis.
Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer.
The Obese Liver Environment Mediates Conversion of NK Cells to a Less Cytotoxic ILC1-Like Phenotype.
Mutations in Hepatitis D Virus Allow It to Escape Detection by CD8(+) T Cells and Evolve at the Population Level.
Peripheral lymph nodes contain migratory and resident innate lymphoid cell populations.
IL-21 Controls ILC3 Cytokine Production and Promotes a Protective Phenotype in a Mouse Model of Colitis.
CD160 serves as a negative regulator of NKT cells in acute hepatic injury.
TIGIT signaling restores suppressor function of Th1 Tregs.
Single-Cell Profiling Defines Transcriptomic Signatures Specific to Tumor-Reactive versus Virus-Responsive CD4(+) T Cells.
Ezh2 controls development of natural killer T cells, which cause spontaneous asthma-like pathology.
CD24(+) Cell Depletion Permits Effective Enrichment of Thymic iNKT Cells While Preserving Their Subset Composition.
FCRL5(+) Memory B Cells Exhibit Robust Recall Responses.
Rapid loss of group 1 innate lymphoid cells during blood stage Plasmodium infection.
Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate.
TRPM2 Exacerbates Central Nervous System Inflammation in Experimental Autoimmune Encephalomyelitis by Increasing Production of CXCL2 Chemokines.
Nitric oxide dependent signaling via cyclic GMP in dendritic cells regulates migration and T-cell polarization.
Fas ligand promotes an inducible TLR-dependent model of cutaneous lupus-like inflammation.
TBK-binding protein 1 regulates IL-15-induced autophagy and NKT cell survival.
Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells.
Regulation of chitinase-3-like-1 in T cell elicits Th1 and cytotoxic responses to inhibit lung metastasis.
Conventional NK cells and ILC1 are partially ablated in the livers of Ncr1 (iCre)Tbx21 (fl/fl) mice.
Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APP(SWE)/PS1(ΔE9) murine model of Alzheimer's disease.
Resistance to TGFβ suppression and improved anti-tumor responses in CD8(+) T cells lacking PTPN22.
NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma.
The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage.
A non-canonical function of Ezh2 preserves immune homeostasis.
Deficiency of N-myristoylation reveals calcineurin activity as regulator of IFN-γ-producing γδ T cells.
Phenotypic and functional characterization of the major lymphocyte populations in the fruit-eating bat Pteropus alecto.
B-cell anergy induces a Th17 shift in a novel B lymphocyte transgenic NOD mouse model, the 116C-NOD mouse.
CXCL16-positive dendritic cells enhance invariant natural killer T cell-dependent IFNγ production and tumor control.
Heat-killed Staphylococcus aureus reduces atherosclerosis by inducing anti-inflammatory macrophages.
The cytotoxic T cell proteome and its shaping by the kinase mTOR.
Regulation of Asymmetric Division by Atypical Protein Kinase C Influences Early Specification of CD8(+) T Lymphocyte Fates.
Human Head and Neck Squamous Cell Carcinoma-Associated Semaphorin 4D Induces Expansion of Myeloid-Derived Suppressor Cells.
Collapse of Cytolytic Potential in SIV-Specific CD8+ T Cells Following Acute SIV Infection in Rhesus Macaques.
Conditions for the generation of cytotoxic CD4(+) Th cells that enhance CD8(+) CTL-mediated tumor regression.
Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche.
Ndfip-mediated degradation of Jak1 tunes cytokine signalling to limit expansion of CD4+ effector T cells.
IL-22-Expressing Murine Lymphocytes Display Plasticity and Pathogenicity in Reporter Mice.
Intranasal Administration of Lentiviral miR-135a Regulates Mast Cell and Allergen-Induced Inflammation by Targeting GATA-3.
Th1-Like ICOS+ Foxp3+ Treg Cells Preferentially Express CXCR3 and Home to β-Islets during Pre-Diabetes in BDC2.5 NOD Mice.
NKp46+ Innate Lymphoid Cells Dampen Vaginal CD8 T Cell Responses following Local Immunization with a Cholera Toxin-Based Vaccine.
Co-potentiation of antigen recognition: A mechanism to boost weak T cell responses and provide immunotherapy in vivo.
PI3Kδ Regulates the Magnitude of CD8+ T Cell Responses after Challenge with Listeria monocytogenes.
Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection.
mTORC1 and mTORC2 selectively regulate CD8⁺ T cell differentiation.
Coupling of T cell receptor specificity to natural killer T cell development by bivalent histone H3 methylation.
Trained immunity in newborn infants of HBV-infected mothers.
Regulation of asymmetric division and CD8+ T lymphocyte fate specification by protein kinase Cζ and protein kinase Cλ/ι.
CD155 (PVR/Necl5) mediates a costimulatory signal in CD4+ T cells and regulates allergic inflammation.
T follicular helper, but not Th1, cell differentiation in the absence of conventional dendritic cells.
Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut.
Dendritic Cell-Based Vaccines that Utilize Myeloid Rather than Plasmacytoid Cells Offer a Superior Survival Advantage in Malignant Glioma.
Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway.
Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue.
CCR7-dependent trafficking of RORγ⁺ ILCs creates a unique microenvironment within mucosal draining lymph nodes.
Chronic lymphocytic leukemia cells express CD38 in response to Th1 cell-derived IFN-γ by a T-bet-dependent mechanism.
OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence.
PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness.
The transduction pattern of IL-12-encoding lentiviral vectors shapes the immunological outcome.
T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate.
Reduced B lymphoid kinase (Blk) expression enhances proinflammatory cytokine production and induces nephrosis in C57BL/6-lpr/lpr mice.
Geometrically controlled asymmetric division of CD4+ T cells studied by immunological synapse arrays.
CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection.
The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells.
The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex.
Islet antigen-specific Th17 cells can induce TNF-α-dependent autoimmune diabetes.
Cutting edge: Failure of antigen-specific CD4+ T cell recruitment to the kidney during systemic candidiasis.
HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells.
CD4CD8αα lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease.
Alterations in regulatory T cells induced by specific oligosaccharides improve vaccine responsiveness in mice.
Mitral and tufted cells are potential cellular targets of nitration in the olfactory bulb of aged mice.
Peptide vaccination in Montanide adjuvant induces and GM-CSF increases CXCR3 and cutaneous lymphocyte antigen expression by tumor antigen-specific CD8 T cells.
Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans.
Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals.
Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses.
Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells.
Transcutaneous vaccination via laser microporation.
TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation.
Regulating mammalian target of rapamycin to tune vaccination-induced CD8(+) T cell responses for tumor immunity.
Increase in Th17 and T-reg lymphocytes and decrease of IL22 correlate with the recovery phase of acute EAE in rat.
Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer.
CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation.
TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B.
The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF.
Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage.
Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma.
Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells.
Differential immunological phenotypes are exhibited after scald and flame burns.
Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa.
Kalim KW, Yang JQ, Modur V, Nguyen P, Li Y, Zheng Y, Guo F
Frontiers in immunology 2021;12:726393
Frontiers in immunology 2021;12:726393
Immune cell phenotypes associated with disease severity and long-term neutralizing antibody titers after natural dengue virus infection.
Rouers A, Chng MHY, Lee B, Rajapakse MP, Kaur K, Toh YX, Sathiakumar D, Loy T, Thein TL, Lim VWX, Singhal A, Yeo TW, Leo YS, Vora KA, Casimiro D, Lim B, Tucker-Kellogg L, Rivino L, Newell EW, Fink K
Cell reports. Medicine 2021 May 18;2(5):100278
Cell reports. Medicine 2021 May 18;2(5):100278
Role of nuclear localization in the regulation and function of T-bet and Eomes in exhausted CD8 T cells.
McLane LM, Ngiow SF, Chen Z, Attanasio J, Manne S, Ruthel G, Wu JE, Staupe RP, Xu W, Amaravadi RK, Xu X, Karakousis GC, Mitchell TC, Schuchter LM, Huang AC, Freedman BD, Betts MR, Wherry EJ
Cell reports 2021 May 11;35(6):109120
Cell reports 2021 May 11;35(6):109120
Heterologous arenavirus vector prime-boost overrules self-tolerance for efficient tumor-specific CD8 T cell attack.
Bonilla WV, Kirchhammer N, Marx AF, Kallert SM, Krzyzaniak MA, Lu M, Darbre S, Schmidt S, Raguz J, Berka U, Vincenti I, Pauzuolis M, Kerber R, Hoepner S, Günther S, Magnus C, Merkler D, Orlinger KK, Zippelius A, Pinschewer DD
Cell reports. Medicine 2021 Mar 16;2(3):100209
Cell reports. Medicine 2021 Mar 16;2(3):100209
STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer.
Lee SJ, Yang H, Kim WR, Lee YS, Lee WS, Kong SJ, Lee HJ, Kim JH, Cheon J, Kang B, Chon HJ, Kim C
Journal for immunotherapy of cancer 2021 Jun;9(6)
Journal for immunotherapy of cancer 2021 Jun;9(6)
Culture, expansion, and flow-cytometry-based functional analysis of pteropid bat MR1-restricted unconventional T cells.
Sia WR, Hey YY, Foo R, Wang LF, Leeansyah E
STAR protocols 2021 Jun 18;2(2):100487
STAR protocols 2021 Jun 18;2(2):100487
Francisella tularensis induces Th1 like MAIT cells conferring protection against systemic and local infection.
Zhao Z, Wang H, Shi M, Zhu T, Pediongco T, Lim XY, Meehan BS, Nelson AG, Fairlie DP, Mak JYW, Eckle SBG, de Lima Moreira M, Tumpach C, Bramhall M, Williams CG, Lee HJ, Haque A, Evrard M, Rossjohn J, McCluskey J, Corbett AJ, Chen Z
Nature communications 2021 Jul 16;12(1):4355
Nature communications 2021 Jul 16;12(1):4355
Splicing factor SRSF1 limits IFN-γ production via RhoH and ameliorates experimental nephritis.
Katsuyama T, Li H, Krishfield SM, Kyttaris VC, Moulton VR
Rheumatology (Oxford, England) 2021 Jan 5;60(1):420-429
Rheumatology (Oxford, England) 2021 Jan 5;60(1):420-429
Targeting Phosphatidylserine Enhances the Anti-tumor Response to Tumor-Directed Radiation Therapy in a Preclinical Model of Melanoma.
Budhu S, Giese R, Gupta A, Fitzgerald K, Zappasodi R, Schad S, Hirschhorn D, Campesato LF, De Henau O, Gigoux M, Liu C, Mazo G, Deng L, Barker CA, Wolchok JD, Merghoub T
Cell reports 2021 Jan 12;34(2):108620
Cell reports 2021 Jan 12;34(2):108620
ADAM12 is a costimulatory molecule that determines Th1 cell fate and mediates tissue inflammation.
Liu Y, Bockermann R, Hadi M, Safari I, Carrion B, Kveiborg M, Issazadeh-Navikas S
Cellular & molecular immunology 2021 Aug;18(8):1904-1919
Cellular & molecular immunology 2021 Aug;18(8):1904-1919
Imbalance between T helper 1 and regulatory T cells plays a detrimental role in experimental Parkinson's disease in mice.
Li W, Luo Y, Xu H, Ma Q, Yao Q
The Journal of international medical research 2021 Apr;49(4):300060521998471
The Journal of international medical research 2021 Apr;49(4):300060521998471
Th2 Biased Immunity With Altered B Cell Profiles in Circulation of Patients With Sporotrichosis Caused by Sporothrix globosa.
Zu J, Yao L, Song Y, Cui Y, Guan M, Chen R, Zhen Y, Li S
Frontiers in immunology 2020;11:570888
Frontiers in immunology 2020;11:570888
Mir-124 Attenuates STAT3-Mediated TH17 Differentiation in Colitis-Driven Colon Cancer.
Lin S, Liu Q, Wen J, Bai K, Guo Y, Wang J
Frontiers in oncology 2020;10:570128
Frontiers in oncology 2020;10:570128
MiR-1165-3p Suppresses Th2 Differentiation via Targeting IL-13 and PPM1A in a Mouse Model of Allergic Airway Inflammation.
Wang Z, Ji N, Chen Z, Sun Z, Wu C, Yu W, Hu F, Huang M, Zhang M
Allergy, asthma & immunology research 2020 Sep;12(5):859-876
Allergy, asthma & immunology research 2020 Sep;12(5):859-876
Programming Multifaceted Pulmonary T Cell Immunity by Combination Adjuvants.
Marinaik CB, Kingstad-Bakke B, Lee W, Hatta M, Sonsalla M, Larsen A, Neldner B, Gasper DJ, Kedl RM, Kawaoka Y, Suresh M
Cell reports. Medicine 2020 Sep 22;1(6):100095
Cell reports. Medicine 2020 Sep 22;1(6):100095
CD19-CAR-T Cells Bearing a KIR/PD-1-Based Inhibitory CAR Eradicate CD19(+)HLA-C1(-) Malignant B Cells While Sparing CD19(+)HLA-C1(+) Healthy B Cells.
Tao L, Farooq MA, Gao Y, Zhang L, Niu C, Ajmal I, Zhou Y, He C, Zhao G, Yao J, Liu M, Jiang W
Cancers 2020 Sep 13;12(9)
Cancers 2020 Sep 13;12(9)
Intestinal vitamin D receptor knockout protects from oxazolone-induced colitis.
Shi Y, Liu Z, Cui X, Zhao Q, Liu T
Cell death & disease 2020 Jun 15;11(6):461
Cell death & disease 2020 Jun 15;11(6):461
Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer.
Wang S, Qu Y, Xia P, Chen Y, Zhu X, Zhang J, Wang G, Tian Y, Ying J, Fan Z
Cell research 2020 Jul;30(7):610-622
Cell research 2020 Jul;30(7):610-622
The Obese Liver Environment Mediates Conversion of NK Cells to a Less Cytotoxic ILC1-Like Phenotype.
Cuff AO, Sillito F, Dertschnig S, Hall A, Luong TV, Chakraverty R, Male V
Frontiers in immunology 2019;10:2180
Frontiers in immunology 2019;10:2180
Mutations in Hepatitis D Virus Allow It to Escape Detection by CD8(+) T Cells and Evolve at the Population Level.
Karimzadeh H, Kiraithe MM, Oberhardt V, Salimi Alizei E, Bockmann J, Schulze Zur Wiesch J, Budeus B, Hoffmann D, Wedemeyer H, Cornberg M, Krawczyk A, Rashidi-Alavijeh J, Rodríguez-Frías F, Casillas R, Buti M, Smedile A, Alavian SM, Heinold A, Emmerich F, Panning M, Gostick E, Price DA, Timm J, Hofmann M, Raziorrouh B, Thimme R, Protzer U, Roggendorf M, Neumann-Haefelin C
Gastroenterology 2019 May;156(6):1820-1833
Gastroenterology 2019 May;156(6):1820-1833
Peripheral lymph nodes contain migratory and resident innate lymphoid cell populations.
Dutton EE, Gajdasik DW, Willis C, Fiancette R, Bishop EL, Camelo A, Sleeman MA, Coccia M, Didierlaurent AM, Tomura M, Pilataxi F, Morehouse CA, Carlesso G, Withers DR
Science immunology 2019 May 31;4(35)
Science immunology 2019 May 31;4(35)
IL-21 Controls ILC3 Cytokine Production and Promotes a Protective Phenotype in a Mouse Model of Colitis.
Poholek CH, Dulson SJ, Zajac AJ, Harrington LE
ImmunoHorizons 2019 Jun 4;3(6):194-202
ImmunoHorizons 2019 Jun 4;3(6):194-202
CD160 serves as a negative regulator of NKT cells in acute hepatic injury.
Kim TJ, Park G, Kim J, Lim SA, Kim J, Im K, Shin MH, Fu YX, Del Rio ML, Rodriguez-Barbosa JI, Yee C, Suh KS, Kim SJ, Ha SJ, Lee KM
Nature communications 2019 Jul 22;10(1):3258
Nature communications 2019 Jul 22;10(1):3258
TIGIT signaling restores suppressor function of Th1 Tregs.
Lucca LE, Axisa PP, Singer ER, Nolan NM, Dominguez-Villar M, Hafler DA
JCI insight 2019 Feb 7;4(3)
JCI insight 2019 Feb 7;4(3)
Single-Cell Profiling Defines Transcriptomic Signatures Specific to Tumor-Reactive versus Virus-Responsive CD4(+) T Cells.
Magen A, Nie J, Ciucci T, Tamoutounour S, Zhao Y, Mehta M, Tran B, McGavern DB, Hannenhalli S, Bosselut R
Cell reports 2019 Dec 3;29(10):3019-3032.e6
Cell reports 2019 Dec 3;29(10):3019-3032.e6
Ezh2 controls development of natural killer T cells, which cause spontaneous asthma-like pathology.
Tumes D, Hirahara K, Papadopoulos M, Shinoda K, Onodera A, Kumagai J, Yip KH, Pant H, Kokubo K, Kiuchi M, Aoki A, Obata-Ninomiya K, Tokoyoda K, Endo Y, Kimura MY, Nakayama T
The Journal of allergy and clinical immunology 2019 Aug;144(2):549-560.e10
The Journal of allergy and clinical immunology 2019 Aug;144(2):549-560.e10
CD24(+) Cell Depletion Permits Effective Enrichment of Thymic iNKT Cells While Preserving Their Subset Composition.
Park JY, Kwon J, Kim EY, Fink J, Kim HK, Park JH
Immune network 2019 Apr;19(2):e14
Immune network 2019 Apr;19(2):e14
FCRL5(+) Memory B Cells Exhibit Robust Recall Responses.
Kim CC, Baccarella AM, Bayat A, Pepper M, Fontana MF
Cell reports 2019 Apr 30;27(5):1446-1460.e4
Cell reports 2019 Apr 30;27(5):1446-1460.e4
Rapid loss of group 1 innate lymphoid cells during blood stage Plasmodium infection.
Ng SS, Souza-Fonseca-Guimaraes F, Rivera FL, Amante FH, Kumar R, Gao Y, Sheel M, Beattie L, Montes de Oca M, Guillerey C, Edwards CL, Faleiro RJ, Frame T, Bunn PT, Vivier E, Godfrey DI, Pellicci DG, Lopez JA, Andrews KT, Huntington ND, Smyth MJ, McCarthy J, Engwerda CR
Clinical & translational immunology 2018;7(1):e1003
Clinical & translational immunology 2018;7(1):e1003
Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate.
Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, Adhikary T, Visekruna A
Scientific reports 2018 Sep 26;8(1):14430
Scientific reports 2018 Sep 26;8(1):14430
TRPM2 Exacerbates Central Nervous System Inflammation in Experimental Autoimmune Encephalomyelitis by Increasing Production of CXCL2 Chemokines.
Tsutsui M, Hirase R, Miyamura S, Nagayasu K, Nakagawa T, Mori Y, Shirakawa H, Kaneko S
The Journal of neuroscience : the official journal of the Society for Neuroscience 2018 Sep 26;38(39):8484-8495
The Journal of neuroscience : the official journal of the Society for Neuroscience 2018 Sep 26;38(39):8484-8495
Nitric oxide dependent signaling via cyclic GMP in dendritic cells regulates migration and T-cell polarization.
Gnipp S, Mergia E, Puschkarow M, Bufe A, Koesling D, Peters M
Scientific reports 2018 Jul 20;8(1):10969
Scientific reports 2018 Jul 20;8(1):10969
Fas ligand promotes an inducible TLR-dependent model of cutaneous lupus-like inflammation.
Mande P, Zirak B, Ko WC, Taravati K, Bride KL, Brodeur TY, Deng A, Dresser K, Jiang Z, Ettinger R, Fitzgerald KA, Rosenblum MD, Harris JE, Marshak-Rothstein A
The Journal of clinical investigation 2018 Jul 2;128(7):2966-2978
The Journal of clinical investigation 2018 Jul 2;128(7):2966-2978
TBK-binding protein 1 regulates IL-15-induced autophagy and NKT cell survival.
Zhu L, Xie X, Zhang L, Wang H, Jie Z, Zhou X, Shi J, Zhao S, Zhang B, Cheng X, Sun SC
Nature communications 2018 Jul 18;9(1):2812
Nature communications 2018 Jul 18;9(1):2812
Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells.
Gaya M, Barral P, Burbage M, Aggarwal S, Montaner B, Warren Navia A, Aid M, Tsui C, Maldonado P, Nair U, Ghneim K, Fallon PG, Sekaly RP, Barouch DH, Shalek AK, Bruckbauer A, Strid J, Batista FD
Cell 2018 Jan 25;172(3):517-533.e20
Cell 2018 Jan 25;172(3):517-533.e20
Regulation of chitinase-3-like-1 in T cell elicits Th1 and cytotoxic responses to inhibit lung metastasis.
Kim DH, Park HJ, Lim S, Koo JH, Lee HG, Choi JO, Oh JH, Ha SJ, Kang MJ, Lee CM, Lee CG, Elias JA, Choi JM
Nature communications 2018 Feb 5;9(1):503
Nature communications 2018 Feb 5;9(1):503
Conventional NK cells and ILC1 are partially ablated in the livers of Ncr1 (iCre)Tbx21 (fl/fl) mice.
Cuff AO, Male V
Wellcome open research 2017;2:39
Wellcome open research 2017;2:39
Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APP(SWE)/PS1(ΔE9) murine model of Alzheimer's disease.
Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, Oyler-Castrillo P, Zhang X, Musch MW, Shen X, Jabri B, Chang EB, Tanzi RE, Sisodia SS
Scientific reports 2017 Sep 5;7(1):10411
Scientific reports 2017 Sep 5;7(1):10411
Resistance to TGFβ suppression and improved anti-tumor responses in CD8(+) T cells lacking PTPN22.
Brownlie RJ, Garcia C, Ravasz M, Zehn D, Salmond RJ, Zamoyska R
Nature communications 2017 Nov 7;8(1):1343
Nature communications 2017 Nov 7;8(1):1343
NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma.
Daley D, Mani VR, Mohan N, Akkad N, Pandian GSDB, Savadkar S, Lee KB, Torres-Hernandez A, Aykut B, Diskin B, Wang W, Farooq MS, Mahmud AI, Werba G, Morales EJ, Lall S, Wadowski BJ, Rubin AG, Berman ME, Narayanan R, Hundeyin M, Miller G
The Journal of experimental medicine 2017 Jun 5;214(6):1711-1724
The Journal of experimental medicine 2017 Jun 5;214(6):1711-1724
The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage.
Shan Q, Zeng Z, Xing S, Li F, Hartwig SM, Gullicksrud JA, Kurup SP, Van Braeckel-Budimir N, Su Y, Martin MD, Varga SM, Taniuchi I, Harty JT, Peng W, Badovinac VP, Xue HH
Nature immunology 2017 Aug;18(8):931-939
Nature immunology 2017 Aug;18(8):931-939
A non-canonical function of Ezh2 preserves immune homeostasis.
Vasanthakumar A, Xu D, Lun AT, Kueh AJ, van Gisbergen KP, Iannarella N, Li X, Yu L, Wang D, Williams BR, Lee SC, Majewski IJ, Godfrey DI, Smyth GK, Alexander WS, Herold MJ, Kallies A, Nutt SL, Allan RS
EMBO reports 2017 Apr;18(4):619-631
EMBO reports 2017 Apr;18(4):619-631
Deficiency of N-myristoylation reveals calcineurin activity as regulator of IFN-γ-producing γδ T cells.
Rampoldi F, Brunk F, Bonrouhi M, Federico G, Krunic D, Porubsky S, Gröne HJ, Popovic ZV
Journal of leukocyte biology 2017 Apr;101(4):1005-1014
Journal of leukocyte biology 2017 Apr;101(4):1005-1014
Phenotypic and functional characterization of the major lymphocyte populations in the fruit-eating bat Pteropus alecto.
Martínez Gómez JM, Periasamy P, Dutertre CA, Irving AT, Ng JH, Crameri G, Baker ML, Ginhoux F, Wang LF, Alonso S
Scientific reports 2016 Nov 24;6:37796
Scientific reports 2016 Nov 24;6:37796
B-cell anergy induces a Th17 shift in a novel B lymphocyte transgenic NOD mouse model, the 116C-NOD mouse.
Carrascal J, Carrillo J, Arpa B, Egia-Mendikute L, Rosell-Mases E, Pujol-Autonell I, Planas R, Mora C, Mauricio D, Ampudia RM, Vives-Pi M, Verdaguer J
European journal of immunology 2016 Mar;46(3):593-608
European journal of immunology 2016 Mar;46(3):593-608
CXCL16-positive dendritic cells enhance invariant natural killer T cell-dependent IFNγ production and tumor control.
Veinotte L, Gebremeskel S, Johnston B
Oncoimmunology 2016 Jun;5(6):e1160979
Oncoimmunology 2016 Jun;5(6):e1160979
Heat-killed Staphylococcus aureus reduces atherosclerosis by inducing anti-inflammatory macrophages.
Frodermann V, van Duijn J, van Puijvelde GH, van Santbrink PJ, Lagraauw HM, de Vries MR, Quax PH, Bot I, Foks AC, de Jager SC, Kuiper J
Journal of internal medicine 2016 Jun;279(6):592-605
Journal of internal medicine 2016 Jun;279(6):592-605
The cytotoxic T cell proteome and its shaping by the kinase mTOR.
Hukelmann JL, Anderson KE, Sinclair LV, Grzes KM, Murillo AB, Hawkins PT, Stephens LR, Lamond AI, Cantrell DA
Nature immunology 2016 Jan;17(1):104-12
Nature immunology 2016 Jan;17(1):104-12
Regulation of Asymmetric Division by Atypical Protein Kinase C Influences Early Specification of CD8(+) T Lymphocyte Fates.
Metz PJ, Lopez J, Kim SH, Akimoto K, Ohno S, Chang JT
Scientific reports 2016 Jan 14;6:19182
Scientific reports 2016 Jan 14;6:19182
Human Head and Neck Squamous Cell Carcinoma-Associated Semaphorin 4D Induces Expansion of Myeloid-Derived Suppressor Cells.
Younis RH, Han KL, Webb TJ
Journal of immunology (Baltimore, Md. : 1950) 2016 Feb 1;196(3):1419-29
Journal of immunology (Baltimore, Md. : 1950) 2016 Feb 1;196(3):1419-29
Collapse of Cytolytic Potential in SIV-Specific CD8+ T Cells Following Acute SIV Infection in Rhesus Macaques.
Roberts ER, Carnathan DG, Li H, Shaw GM, Silvestri G, Betts MR
PLoS pathogens 2016 Dec;12(12):e1006135
PLoS pathogens 2016 Dec;12(12):e1006135
Conditions for the generation of cytotoxic CD4(+) Th cells that enhance CD8(+) CTL-mediated tumor regression.
Li K, Baird M, Yang J, Jackson C, Ronchese F, Young S
Clinical & translational immunology 2016 Aug;5(8):e95
Clinical & translational immunology 2016 Aug;5(8):e95
Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche.
Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, Palmer DC, Phan AT, Goulding J, Gattinoni L, Goldrath AW, Belkaid Y, Restifo NP
Cell 2016 Aug 25;166(5):1117-1131.e14
Cell 2016 Aug 25;166(5):1117-1131.e14
Ndfip-mediated degradation of Jak1 tunes cytokine signalling to limit expansion of CD4+ effector T cells.
O'Leary CE, Riling CR, Spruce LA, Ding H, Kumar S, Deng G, Liu Y, Seeholzer SH, Oliver PM
Nature communications 2016 Apr 18;7:11226
Nature communications 2016 Apr 18;7:11226
IL-22-Expressing Murine Lymphocytes Display Plasticity and Pathogenicity in Reporter Mice.
Shen W, Hixon JA, McLean MH, Li WQ, Durum SK
Frontiers in immunology 2015;6:662
Frontiers in immunology 2015;6:662
Intranasal Administration of Lentiviral miR-135a Regulates Mast Cell and Allergen-Induced Inflammation by Targeting GATA-3.
Deng YQ, Yang YQ, Wang SB, Li F, Liu MZ, Hua QQ, Tao ZZ
PloS one 2015;10(9):e0139322
PloS one 2015;10(9):e0139322
Th1-Like ICOS+ Foxp3+ Treg Cells Preferentially Express CXCR3 and Home to β-Islets during Pre-Diabetes in BDC2.5 NOD Mice.
Kornete M, Mason ES, Girouard J, Lafferty EI, Qureshi S, Piccirillo CA
PloS one 2015;10(5):e0126311
PloS one 2015;10(5):e0126311
NKp46+ Innate Lymphoid Cells Dampen Vaginal CD8 T Cell Responses following Local Immunization with a Cholera Toxin-Based Vaccine.
Luci C, Bekri S, Bihl F, Pini J, Bourdely P, Nouhen K, Malgogne A, Walzer T, Braud VM, Anjuère F
PloS one 2015;10(12):e0143224
PloS one 2015;10(12):e0143224
Co-potentiation of antigen recognition: A mechanism to boost weak T cell responses and provide immunotherapy in vivo.
Hoffmann MM, Molina-Mendiola C, Nelson AD, Parks CA, Reyes EE, Hansen MJ, Rajagopalan G, Pease LR, Schrum AG, Gil D
Science advances 2015 Oct;1(9):e1500415
Science advances 2015 Oct;1(9):e1500415
PI3Kδ Regulates the Magnitude of CD8+ T Cell Responses after Challenge with Listeria monocytogenes.
Pearce VQ, Bouabe H, MacQueen AR, Carbonaro V, Okkenhaug K
Journal of immunology (Baltimore, Md. : 1950) 2015 Oct 1;195(7):3206-17
Journal of immunology (Baltimore, Md. : 1950) 2015 Oct 1;195(7):3206-17
Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection.
Omilusik KD, Best JA, Yu B, Goossens S, Weidemann A, Nguyen JV, Seuntjens E, Stryjewska A, Zweier C, Roychoudhuri R, Gattinoni L, Bird LM, Higashi Y, Kondoh H, Huylebroeck D, Haigh J, Goldrath AW
The Journal of experimental medicine 2015 Nov 16;212(12):2027-39
The Journal of experimental medicine 2015 Nov 16;212(12):2027-39
mTORC1 and mTORC2 selectively regulate CD8⁺ T cell differentiation.
Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, Delgoffe GM, Powell JD
The Journal of clinical investigation 2015 May;125(5):2090-108
The Journal of clinical investigation 2015 May;125(5):2090-108
Coupling of T cell receptor specificity to natural killer T cell development by bivalent histone H3 methylation.
Dobenecker MW, Kim JK, Marcello J, Fang TC, Prinjha R, Bosselut R, Tarakhovsky A
The Journal of experimental medicine 2015 Mar 9;212(3):297-306
The Journal of experimental medicine 2015 Mar 9;212(3):297-306
Trained immunity in newborn infants of HBV-infected mothers.
Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, Urbani S, Chong YS, Guccione E, Bertoletti A
Nature communications 2015 Mar 25;6:6588
Nature communications 2015 Mar 25;6:6588
Regulation of asymmetric division and CD8+ T lymphocyte fate specification by protein kinase Cζ and protein kinase Cλ/ι.
Metz PJ, Arsenio J, Kakaradov B, Kim SH, Remedios KA, Oakley K, Akimoto K, Ohno S, Yeo GW, Chang JT
Journal of immunology (Baltimore, Md. : 1950) 2015 Mar 1;194(5):2249-59
Journal of immunology (Baltimore, Md. : 1950) 2015 Mar 1;194(5):2249-59
CD155 (PVR/Necl5) mediates a costimulatory signal in CD4+ T cells and regulates allergic inflammation.
Yamashita-Kanemaru Y, Takahashi Y, Wang Y, Tahara-Hanaoka S, Honda S, Bernhardt G, Shibuya A, Shibuya K
Journal of immunology (Baltimore, Md. : 1950) 2015 Jun 15;194(12):5644-53
Journal of immunology (Baltimore, Md. : 1950) 2015 Jun 15;194(12):5644-53
T follicular helper, but not Th1, cell differentiation in the absence of conventional dendritic cells.
Dahlgren MW, Gustafsson-Hedberg T, Livingston M, Cucak H, Alsén S, Yrlid U, Johansson-Lindbom B
Journal of immunology (Baltimore, Md. : 1950) 2015 Jun 1;194(11):5187-99
Journal of immunology (Baltimore, Md. : 1950) 2015 Jun 1;194(11):5187-99
Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut.
Kim MH, Taparowsky EJ, Kim CH
Immunity 2015 Jul 21;43(1):107-19
Immunity 2015 Jul 21;43(1):107-19
Dendritic Cell-Based Vaccines that Utilize Myeloid Rather than Plasmacytoid Cells Offer a Superior Survival Advantage in Malignant Glioma.
Dey M, Chang AL, Miska J, Wainwright DA, Ahmed AU, Balyasnikova IV, Pytel P, Han Y, Tobias A, Zhang L, Qiao J, Lesniak MS
Journal of immunology (Baltimore, Md. : 1950) 2015 Jul 1;195(1):367-76
Journal of immunology (Baltimore, Md. : 1950) 2015 Jul 1;195(1):367-76
Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway.
Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH
Mucosal immunology 2015 Jan;8(1):80-93
Mucosal immunology 2015 Jan;8(1):80-93
Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue.
Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, Godfrey DI, Leadbetter EA, Sant'Angelo DB, von Andrian U, Brenner MB
Nature immunology 2015 Jan;16(1):85-95
Nature immunology 2015 Jan;16(1):85-95
CCR7-dependent trafficking of RORγ⁺ ILCs creates a unique microenvironment within mucosal draining lymph nodes.
Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, Filbey KJ, Maizels RM, Hepworth MR, Sonnenberg GF, Milling S, Withers DR
Nature communications 2015 Jan 9;6:5862
Nature communications 2015 Jan 9;6:5862
Chronic lymphocytic leukemia cells express CD38 in response to Th1 cell-derived IFN-γ by a T-bet-dependent mechanism.
Bürgler S, Gimeno A, Parente-Ribes A, Wang D, Os A, Devereux S, Jebsen P, Bogen B, Tjønnfjord GE, Munthe LA
Journal of immunology (Baltimore, Md. : 1950) 2015 Jan 15;194(2):827-35
Journal of immunology (Baltimore, Md. : 1950) 2015 Jan 15;194(2):827-35
OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence.
Buchan S, Manzo T, Flutter B, Rogel A, Edwards N, Zhang L, Sivakumaran S, Ghorashian S, Carpenter B, Bennett C, Freeman GJ, Sykes M, Croft M, Al-Shamkhani A, Chakraverty R
Journal of immunology (Baltimore, Md. : 1950) 2015 Jan 1;194(1):125-133
Journal of immunology (Baltimore, Md. : 1950) 2015 Jan 1;194(1):125-133
PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness.
Yang M, Li D, Chang Z, Yang Z, Tian Z, Dong Z
The Journal of experimental medicine 2015 Feb 9;212(2):253-65
The Journal of experimental medicine 2015 Feb 9;212(2):253-65
The transduction pattern of IL-12-encoding lentiviral vectors shapes the immunological outcome.
Goyvaerts C, Broos K, Escors D, Heirman C, Raes G, De Baetselier P, Thielemans K, Breckpot K
European journal of immunology 2015 Dec;45(12):3351-61
European journal of immunology 2015 Dec;45(12):3351-61
T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate.
Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR
Immunity 2015 Dec 15;43(6):1101-11
Immunity 2015 Dec 15;43(6):1101-11
Reduced B lymphoid kinase (Blk) expression enhances proinflammatory cytokine production and induces nephrosis in C57BL/6-lpr/lpr mice.
Samuelson EM, Laird RM, Papillion AM, Tatum AH, Princiotta MF, Hayes SM
PloS one 2014;9(3):e92054
PloS one 2014;9(3):e92054
Geometrically controlled asymmetric division of CD4+ T cells studied by immunological synapse arrays.
Jung HR, Song KH, Chang JT, Doh J
PloS one 2014;9(3):e91926
PloS one 2014;9(3):e91926
CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection.
Linterman MA, Denton AE, Divekar DP, Zvetkova I, Kane L, Ferreira C, Veldhoen M, Clare S, Dougan G, Espéli M, Smith KG
eLife 2014 Oct 27;3
eLife 2014 Oct 27;3
The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells.
Tang W, Wang H, Claudio E, Tassi I, Ha HL, Saret S, Siebenlist U
Immunity 2014 Oct 16;41(4):555-66
Immunity 2014 Oct 16;41(4):555-66
The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex.
Heikamp EB, Patel CH, Collins S, Waickman A, Oh MH, Sun IH, Illei P, Sharma A, Naray-Fejes-Toth A, Fejes-Toth G, Misra-Sen J, Horton MR, Powell JD
Nature immunology 2014 May;15(5):457-64
Nature immunology 2014 May;15(5):457-64
Islet antigen-specific Th17 cells can induce TNF-α-dependent autoimmune diabetes.
Li CR, Mueller EE, Bradley LM
Journal of immunology (Baltimore, Md. : 1950) 2014 Feb 15;192(4):1425-32
Journal of immunology (Baltimore, Md. : 1950) 2014 Feb 15;192(4):1425-32
Cutting edge: Failure of antigen-specific CD4+ T cell recruitment to the kidney during systemic candidiasis.
Drummond RA, Wallace C, Reid DM, Way SS, Kaplan DH, Brown GD
Journal of immunology (Baltimore, Md. : 1950) 2014 Dec 1;193(11):5381-5
Journal of immunology (Baltimore, Md. : 1950) 2014 Dec 1;193(11):5381-5
HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells.
Araya N, Sato T, Ando H, Tomaru U, Yoshida M, Coler-Reilly A, Yagishita N, Yamauchi J, Hasegawa A, Kannagi M, Hasegawa Y, Takahashi K, Kunitomo Y, Tanaka Y, Nakajima T, Nishioka K, Utsunomiya A, Jacobson S, Yamano Y
The Journal of clinical investigation 2014 Aug;124(8):3431-42
The Journal of clinical investigation 2014 Aug;124(8):3431-42
CD4CD8αα lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease.
Sarrabayrouse G, Bossard C, Chauvin JM, Jarry A, Meurette G, Quévrain E, Bridonneau C, Preisser L, Asehnoune K, Labarrière N, Altare F, Sokol H, Jotereau F
PLoS biology 2014 Apr;12(4):e1001833
PLoS biology 2014 Apr;12(4):e1001833
Alterations in regulatory T cells induced by specific oligosaccharides improve vaccine responsiveness in mice.
Schijf MA, Kerperien J, Bastiaans J, Szklany K, Meerding J, Hofman G, Boon L, van Wijk F, Garssen J, Van't Land B
PloS one 2013;8(9):e75148
PloS one 2013;8(9):e75148
Mitral and tufted cells are potential cellular targets of nitration in the olfactory bulb of aged mice.
Yang MJ, Sim S, Jeon JH, Jeong E, Kim HC, Park YJ, Kim IB
PloS one 2013;8(3):e59673
PloS one 2013;8(3):e59673
Peptide vaccination in Montanide adjuvant induces and GM-CSF increases CXCR3 and cutaneous lymphocyte antigen expression by tumor antigen-specific CD8 T cells.
Clancy-Thompson E, King LK, Nunnley LD, Mullins IM, Slingluff CL Jr, Mullins DW
Cancer immunology research 2013 Nov;1(5):332-9
Cancer immunology research 2013 Nov;1(5):332-9
Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans.
Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 1;190(5):2001-8
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 1;190(5):2001-8
Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals.
Bailis W, Yashiro-Ohtani Y, Fang TC, Hatton RD, Weaver CT, Artis D, Pear WS
Immunity 2013 Jul 25;39(1):148-59
Immunity 2013 Jul 25;39(1):148-59
Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses.
Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J
World journal of gastroenterology 2013 Aug 7;19(29):4702-17
World journal of gastroenterology 2013 Aug 7;19(29):4702-17
Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA
Nature 2013 Apr 25;496(7446):518-22
Nature 2013 Apr 25;496(7446):518-22
Transcutaneous vaccination via laser microporation.
Weiss R, Hessenberger M, Kitzmüller S, Bach D, Weinberger EE, Krautgartner WD, Hauser-Kronberger C, Malissen B, Boehler C, Kalia YN, Thalhamer J, Scheiblhofer S
Journal of controlled release : official journal of the Controlled Release Society 2012 Sep 10;162(2):391-9
Journal of controlled release : official journal of the Controlled Release Society 2012 Sep 10;162(2):391-9
TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation.
Zhang N, Bevan MJ
Nature immunology 2012 May 27;13(7):667-73
Nature immunology 2012 May 27;13(7):667-73
Regulating mammalian target of rapamycin to tune vaccination-induced CD8(+) T cell responses for tumor immunity.
Li Q, Rao R, Vazzana J, Goedegebuure P, Odunsi K, Gillanders W, Shrikant PA
Journal of immunology (Baltimore, Md. : 1950) 2012 Apr 1;188(7):3080-7
Journal of immunology (Baltimore, Md. : 1950) 2012 Apr 1;188(7):3080-7
Increase in Th17 and T-reg lymphocytes and decrease of IL22 correlate with the recovery phase of acute EAE in rat.
Almolda B, Costa M, Montoya M, González B, Castellano B
PloS one 2011;6(11):e27473
PloS one 2011;6(11):e27473
Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer.
Haabeth OA, Lorvik KB, Hammarström C, Donaldson IM, Haraldsen G, Bogen B, Corthay A
Nature communications 2011;2:240
Nature communications 2011;2:240
CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation.
Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Ménoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, Adler AJ
Journal of immunology (Baltimore, Md. : 1950) 2011 Oct 1;187(7):3555-64
Journal of immunology (Baltimore, Md. : 1950) 2011 Oct 1;187(7):3555-64
TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B.
Gaddis DE, Michalek SM, Katz J
Journal of immunology (Baltimore, Md. : 1950) 2011 May 15;186(10):5772-83
Journal of immunology (Baltimore, Md. : 1950) 2011 May 15;186(10):5772-83
The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF.
El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A
Nature immunology 2011 Jun;12(6):568-75
Nature immunology 2011 Jun;12(6):568-75
Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage.
Hendricks DW, Fink PJ
Blood 2011 Jan 27;117(4):1239-49
Blood 2011 Jan 27;117(4):1239-49
Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma.
Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA
The Journal of experimental medicine 2010 Mar 15;207(3):651-67
The Journal of experimental medicine 2010 Mar 15;207(3):651-67
Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells.
Ness-Schwickerath KJ, Jin C, Morita CT
Journal of immunology (Baltimore, Md. : 1950) 2010 Jun 15;184(12):7268-80
Journal of immunology (Baltimore, Md. : 1950) 2010 Jun 15;184(12):7268-80
Differential immunological phenotypes are exhibited after scald and flame burns.
Tschöp J, Martignoni A, Reid MD, Adediran SG, Gardner J, Noel GJ, Ogle CK, Neely AN, Caldwell CC
Shock (Augusta, Ga.) 2009 Feb;31(2):157-63
Shock (Augusta, Ga.) 2009 Feb;31(2):157-63
Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa.
Imanguli MM, Swaim WD, League SC, Gress RE, Pavletic SZ, Hakim FT
Blood 2009 Apr 9;113(15):3620-30
Blood 2009 Apr 9;113(15):3620-30
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
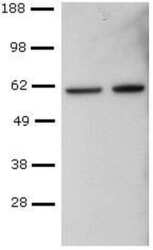
- Experimental details
- CD4+ T cells were sorted from mouse spleen, activated with Anti-Mouse CD3 and Anti-Mouse CD28, followed by culture in Th1-polarizing conditions, and re-activation with PMA and Ionomycin. Lysates from control (left) or PMA and Ionomycin-re-activated (right) cells were probed with Anti-Human/Mouse T-bet Purified at 2 µg/mL, and revealed with Anti-Mouse IgG HRP.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- CD4+ T cells were sorted from mouse spleen, activated with Anti-Mouse CD3 and Anti-Mouse CD28, followed by culture in Th1-polarizing conditions, and re-activation with PMA and Ionomycin. Lysates from control (left) or PMA and Ionomycin-re-activated (right) cells were probed with Anti-Human/Mouse T-bet Purified at 2 µg/mL, and revealed with Anti-Mouse IgG HRP.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- CD4+ T cells were sorted from mouse spleen, activated with Anti-Mouse CD3 and Anti-Mouse CD28, followed by culture in Th1-polarizing conditions, and re-activation with PMA and Ionomycin. Lysates from control (left) or PMA and Ionomycin-re-activated (right) cells were probed with Anti-Human/Mouse T-bet Purified at 2 µg/mL, and revealed with Anti-Mouse IgG HRP.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Knockdown of T-bet was achieved by transfecting NK-92 with T-bet specific siRNAs (Silencer® select Product # s26889, s223839). Western blot analysis (Fig. a) was performed using modified whole cell extracts (1% SDS) from the T-bet knockdown cells (lane 3), non-specific scrambled siRNA transfected cells (lane 2) and untransfected cells (lane 1). The blot was probed with T-bet Monoclonal Antibody (Product # 14-5825-82, 2ug/ml) and Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 0.25µg/ml, 1:4000 dilution). Densitometric analysis of this western blot is shown in histogram (Fig. b). Decrease in signal upon siRNA mediated knock down confirms that antibody is specific to T-bet.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot was performed using Anti-T-bet Monoclonal Antibody (Product # 14-5825-82) and a 58kDa band corresponding to T-bet was observed across cell lines tested and the protein was not expressed in K-562. Modified whole cell extracts (1% SDS) (30 µg lysate) of NK-92 (Lane 1) and K-562 (Lane 2) were electrophoresed using Novex® NuPAGE® 4-12 % Bis-Tris gel (Product # NP0322BOX). Resolved proteins were then transferred onto a nitrocellulose membrane (Product # IB23001) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (2ug/ml) and detected by chemiluminescence with Goat anti-Mouse IgG (H+L), Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000 dilution) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescence analysis of T-bet was performed using 70% confluent log phase NK-92 cells. The cells were fixed with 4% paraformaldehyde for 10 Minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with T-bet Monoclonal Antibody (Product # 14-5825-82) at 5 µg/mL in 0.1% BSA, incubated at 4 degree Celsius overnight and then labeled with Goat anti-Mouse IgG (H+L), Superclonal™ Recombinant Secondary Antibody, Alexa Fluor 488 (Product # A28175) at a dilution of 1:2,000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415). Panel d represents the merged image showing nuclear localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 1 wk ABX gvg-treated APP SWE /PS1 DeltaE9 mice display altered peripheral and brain inflammatory profiles. ( A ) Representative density dot plots of T-bet and Foxp3 intracellular expression in TCRbeta + CD4 + T cell populations isolated from MLN, blood and brain tissue of vehicle and 1 wk ABX gvg-treated APP SWE /PS1 DeltaE9 mice analysed by flow cytometry. Quantified percentages of ( B ) Foxp3 + and ( C ) T-bet + CD4 + T cells, representative of a T-reg and Th1 T cell phenotype respectively, are expressed relative to total live CD4 + T cell counts ( n = 5-6, *p < 0.05, **p < 0.01, un-paired two-tailed Student's t -test). ( D ) Immunoblot-based array of inflammatory mediators present in the serum of vehicle and 1 wk ABX gvg-treated APP SWE /PS1 DeltaE9 mice ( n = 10 pooled sera). ( E ) Immunoblot-based array of inflammatory mediators present in the CSF of vehicle and 1 wk ABX gvg-treated APP SWE /PS1 DeltaE9 mice ( n = 10 pooled CSF). ( F ) Heat map analysis of inflammatory mediator fold change expression in 1 wk ABX gvg-treated APP SWE /PS1 DeltaE9 mice relative to control. Data are displayed as log e (mean) or mean +- SEM. See Supp. Figs 2 , 4 , 5 , 6 , and statistical Table 2 for additional information.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
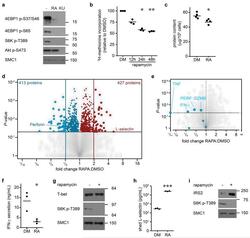
- Figure 4 The mTORC1 regulated CTL proteome (a) Immunoblot analysis of mTORC1/2 substrates in P14 TCR transgenic CTLs cultured with IL-2/IL-12 +- 48 h treatment with either rapamycin or KU-0063794. (b) Protein synthesis was examined by monitoring 3 H-Met incorporation into nascent proteins in CTLs cultured in IL-2/IL-12 and treated with rapamycin for the indicated time. (c) Cellular protein content of CTLs +- 48 h rapamycin. (d, e,) Volcano plots showing fold changes in proteins vs. log-transformed P -values from mass spectrometry analysis of CTLs +- 48 h rapamycin. (d) Total proteins. Known rapamycin sensitive proteins perforin and L-selectin are highlighted. (e) CTL effector molecules. (f) IFN-gamma secretion by CTLs +- 48 h rapamycin measured by ELISA. (h) Immunoblot analysis of T-bet in CTLs +- 48 h rapamycin. (h, i) Validation of up-regulated proteins: (h) ELISA of shed CD62L in cell supernatants prepared from CTLs +- 48 h rapamycin. (i) Immunoblot analysis of IRS2 in CTLs +- 48 h rapamycin. (a, g, i): representive immunoblots of at least three biological replicates. (b, c, f, h): individual data points and means are shown. P -values shown determined by (b): one-way ANOVA (Holm-Sidak) vs. DMSO as control on non-normalized data; (c, f, h): two-tailed Student's t-test. Data based on three (b, f, h) or four (c) biological replicates. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 2 Absence of CD160 does not affect the development of NKT cells. a Lymphocytes from blood, thymus, liver, and spleen in WT and CD160 -/- mice were stained with anti-TCRbeta FITC and PBS57-CD1d tetramer PE and analyzed by flow cytometry. Results are representative of three independent experiments. The absolute number of TCRbeta + PBS57-CD1d Tetramer + NKT cells in the thymus, liver, and spleen was calculated based on the percentage of each population shown in ( n = 3 per group). b Flow cytometry analysis of CD44 and NK1.1 expression among total NKT cells from the thymus of WT and CD160 -/- mice. Results are representative of three independent experiments. The percentages and numbers of thymic NKT cells at stage 0 (NK1.1 - , CD44 - , CD24 + ), stage 1 (NK1.1 - , CD44 - , CD24 - ), stage 2 (NK1.1 - , CD44 + ), and stage 3 (NK1.1 + , CD44 + ), based on flow cytometry analysis. Results are representative of three independent experiments. ( n = 5-6 per group). c Thymic NKT cells were gated on PLZF, T-bet and RORgammat and the percentage and absolute numbers of NKT1, NKT2, and NKT17 were calculated ( n = 5-6 per group). All data are presented as mean +- S.E.M. * P < 0.05, ** P < 0.01, *** P < 0.001 with an unpaired two-tailed T -test
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 5 Lentiviral-mmu-miR-135a treatment influences Th cell polarization. The expression of T-bet and GATA-3 protein in CD4 + T cells was measured in the spleens of normal (control), AR (AR-induced), positive (AR-induced, treated with lentiviral-mmu-miR-135a), and negative (AR-induced, treated with empty lentivirus) mice using flow cytometry. (A) Representative dot plots from each experimental group. The percentages of CD4 + T-bet + T cells (B) and CD4 + GATA-3 + T cells (C) were also calculated. Data are presented as the mean +- SEM. *P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 7. Melanoma Patients Demonstrated an Overall Increase of PS Expression on Immune Subsets in the Blood 4-7 Days after RT Peripheral blood was collected from 7 melanoma patients before and 4-7 days after receiving tumor-directed RT. Freshly isolated PBMCs from each patient were stained for PS expression using annexin V on the day the blood was collected, as described in STAR Methods . (A) Histogram plots of annexin V staining of viable immune cell subsets in PBMCs from a single patient (Pt. 1) pretreatment (Tx) and post-RT. (B) Representative plots gated on live CD3+ CD8+ T cells of annexin V versus caspase-3/7 activity of PBMCs from a single patient pre-RT and 4 days post-RT. FMO, control. (C) Top: individual values for each patient. Bottom: average percentage +- SEM of annexin V+ immune cell subsets pre-RT and post-RT. *p < 0.05, ***p < 0.005. (D) Model summarizing the effects of targeting PS with RT and anti-PD-1 on immune cell activation.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
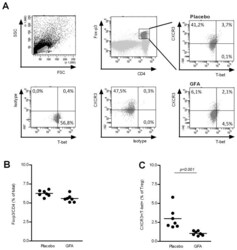
- Figure 4 Treg population in MLN is influenced by the dietary intervention. Cells from MLNs were isolated from mice (n = 7 per group) receiving either placebo or scGOS/lcFOS/pAOS and were labeled with CD4/Foxp3 in combination with CXCR3, T-bet, for flowcytometric analysis (A). Lines represent mean percentages of Tregs in total (CD4 + Foxp3 + T cells) (B), and sub-populations of Tregs including % of CXCR3 + /T-bet + Tregs (C) In addition, individual measurements are indicated through separate dots. Data presented is representative for 3 individual experiments. Statistically significant differences between the groups are indicated in the graphs.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 Cellular localization of 3-NT in the OB of aged mice by double-labeling immunohistochemistry with two neuronal markers, anti-NeuN (A-H) and anti-TBX21 (L-N). A-D: Glomerular layer. A: Small and large 3-NT-labeled puncta (red) are seen within the glomeruli (circles) and in the periglomerular region. B: Numerous periglomerular cells localized in the periglomerular region are labeled with anti-NeuN (green), a neuronal cell marker. C: In this merged image of A and B, few 3-NT labeled puncta are localized in NeuN-labeled periglomerular cells. D: A transmission light micrograph overlaid with C. E-H: Mitral cell layer (MCL) and 2 adjacent regions of the external plexiform layer (EPL) and the inner plexiform layer (IPL). E: Large 3-NT-labeled puncta are clearly seen on the border of the MCL and EPL. F: Many NeuN-labeled granule cells located in the MCL are observed. G: In this merged image of E and F, large 3-NT-labeled puncta are not localized to NeuN-labeled granule cells. H: In a transmission light micrograph overlaid with G, the large 3-NT-labeled puncta are exclusively localized externally to putative NeuN-negative mitral cells (asterisks). The rectangular area is magnified in I-K. I-K: A mitral cell. I: In a transmission light micrograph overlaid with red immunofluorescence, the 3-NT-labeled puncta with triangular shape is clearly seen in the apical portion of a putative mitral cell. J: In the confocal image using the red channel for 3-NT immunoreactivity, the labeled
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Phenotype of artARENA-induced CTLs and their dependence on IL-33-ST2 alarmin signaling (A-F) We immunized BALB/c mice with artPICV-P1A and artLCMV-P1A in homologous or heterologous prime-boost vaccination i.v. on day 0 and day 27. On day 34, we analyzed P1A-Tet-binding and CD62L expression by splenic CD8 + T cells (A; gated on CD8 + B220 - lymphocytes). Unimmunized control mice are shown for comparison in (A) only. Numbers in (A) indicate the percentage of cells in the respective quadrant. Total P1A-Tet + CTLs (B), P1A-specific effector/effector memory CTLs (CD62L lo ; C), and P1A-specific central memory CTLs (CD62L hi ; D) were enumerated in the spleen on day 34. In both subsets of P1A-specific CTLs, CD62L hi and CD62L lo , we determined the surface expression of KLRG1, CX3CR1, CD27, CD43, and CD127 as well as the master transcription factors Tcf-1, Tbet, and Eomes (E). Total numbers of marker-expressing P1A-specific CTLs were enumerated in (F). (A) shows representative FACS plots from individual mice. Symbols in (B)-(D) and (F) represent individual mice, and bars in (B)-(D) and (F) indicate the mean +- SD. Numbers in (A) and (E) indicate the percentage of gated cells (mean +- SD) or the mean fluorescence intensity (MFI +- SD). Means were calculated from six mice per immunization group (A-F) or from three unimmunized controls (A). N = 2. **p < 0.01 by unpaired two-tailed Student''s t test. (G and H) We immunized ST2 -/- and WT mice with artLCMV-E7E6, artPICV-E7E6 or
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1. Patients with Parkinson's disease (PD) and mice with experimental PD exhibit decreased regulatory T (Treg) and increased T helper 1 (Th1) cell numbers in the blood. Treg cells were defined as CD3+CD4+CD25+FoxP3+ cells, whereas Th1 cells were identified as CD3+CD4+T-bet+ cells. (a) Representative plots of Treg and Th1 cells in patients with PD and healthy volunteers (HVs). (b) Representative plots of Treg and Th1 cells in control mice (Saline) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. (c) Patients with PD exhibited lower Treg cell levels and greater Th1 cell levels in the blood than HVs. n = 20. ****, p < 0.0001 by a two-tailed Student's t tests. (d) MPTP-induced experimental PD mice had fewer Treg and more Th1 cells in the circulation than saline-treated mice. n = 9/group in each experiment performed in triplicate. ***, p < 0.001; ****, p < 0.0001, according to Student's t test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1 Altered distribution of Th1, Th2, and Th17 in PBMCs of patients. (A) PBMCs were stained intracellularly with T-bet, GATA-3, and ROR-gammat mAbs after surface staining of CD4 mAb. According to CD4 staining and SSC, CD4 + T cells were gated. The parameters shown in quadrants of the representative graphs are mean frequency of GATA-3 for each group. (B-D) The average percentages of CD4 + T-bet + Th1, CD4 + GATA-3 + Th2, CD4 + ROR-gammat + Th17 are compared between HC (n = 24) and whole patients (n = 50) as well as subgroups of patients (SD, n = 24; LD, n = 26; FF, n = 33; LF, n = 17). Error bars represent mean+-SD. ** P < 0.01, * P < 0.05, and NS P >= 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 3 ADAM12 is essential for T-bet expression in T cells and IFNgamma production. a ADAM12 +/+ and ADAM12 -/- T cells were treated with plate-bound anti-CD3 (5 mug/ml) and anti-ADAM12 (10 mug/ml) for 3 days. Cell-culture supernatants were collected for ELISA. MOG 35-55 -specific T cells treated with MOG 35-55 (50 mug/ml), IL-12 (20 ng/ml), anti-IL-4 (10 mug/ml), and siRNAs (siControl or siADAM12) for 72 h. b FACS dot plots of the gating strategy and T-bet staining in CD4 + T cells. c Quantification of FACS results from b . d IFNgamma production in Th1 cell cultures by ELISA. Graphs in c, d are mean +- SEM from three independent experiments. *** P < 0.001 by Student's t test. Th17 cells polarized in vitro. e , f Representative FACS plots of gating strategy and T-bet- and RORgammat-stained CD4 + T cells. g Quantification of FACS results from a representative experiment--i.e., from two independent experiments. Graphs are mean +- SEM, N = 3. h IFNgamma and IL-17 production in Th17 cell cultures, by ELISA from three independent experiments. ** P < 0.01 by two-way ANOVA with post-Tukey's multiple comparisons test
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
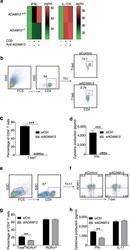
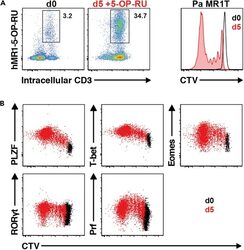
- Figure 13 Pa MR1T cell antigen-specific proliferation assay (A) Representative FACS plots showing frequencies of Pa MR1T cells (defined as CD3 + hMR1-5-OP-RU + ) and CTV dilution on day 0 and day 5 of culture with 5-OP-RU from a single Pa donor. (B) Representative FACS plots showing the expression levels of different transcription factors (PLZF, T-bet, Eomes, and RORgammat) and Perforin against CTV at the two different timepoints. Figure adapted and reprinted with permission from ().
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1. Exhausted CD8 T cells have a high ratio of nuclear Eomes to T-bet that correlates with PD-1 expression during LCMV infection ImageStream analysis was performed on CD8 + T cells from Armstrong-immune (T MEM , blue)- or clone 13 (T EX , red)-infected mice at day 30 p.i. (A) Representative cell images acquired in IDEAS software from an Armstrong-immune mouse (T MEM , left) or chronic clone 13 mouse (T EX, right) are shown. Splenocytes were permeabilized and stained with T-bet (yellow). The location of the nucleus is indicated by DAPI (cyan). (B) Representative ImageStream flow plots displaying T-bet localization in T MEM s or T EX s are shown (left). Bar graphs display the frequency and median fluorescence intensity (MFI) of nuclear T-bet in LCMV-specific H-2D b gp276 + CD8 + T cells (right). (C) Representative cell images acquired in IDEAS software from an Armstrong-immune mouse (T MEM , left) or chronic clone 13 mouse (T EX , right) are shown. Splenocytes were permeabilized and stained with Eomes (magenta). The location of the nucleus is indicated by DAPI (cyan). (D) Representative ImageStream flow plots displaying Eomes localization are shown (left). Bar graphs show the frequency and MFI of nuclear Eomes in LCMV-specific H-2D b gp276 + CD8 + T cells (right). (E) The ratio of the MFI of nuclear Eomes:T-bet in LCMV-specific H-2D b gp276 + T-bet + Eomes + CD8 T cells is shown. (F) A correlation plot displaying the ratio of the MFI of nuclear Eomes:T-bet versus MFI of P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
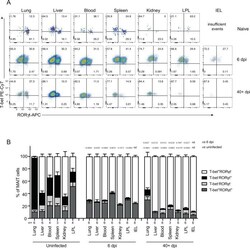
- Fig. 4 MAIT cells were polarized to functional MAIT-1 phenotype upon F. tularensis infection. A Representative flow cytometry plots showing intranuclear staining for T-bet (representing Th1) and RORgammat (Th17) in gated MAIT cells from the liver, lungs, spleen, kidneys, LPL, IEL, and blood of naive and infected mice on 6 and 40+ dpi (41 and 68 dpi in two experiments) with 10 4 CFU F. tularensis LVS i.v. Numbers in quadrants represent cell percentage. It is noteworthy that IEL from naive mice yielded insufficient numbers of MAIT cells for accurate assessment of transcription factor expression and, thus, were omitted from our analysis. B Percentage of MAIT cells expressing combinations of T-bet and RORgammat from the same mice in A . Pooled data from two independent experiments (mean +- SEM, n = 3-6 mice per group, as indicated). One-way ANOVA with Tukey's multiple comparisons test was performed on MAIT-1% in each organ (except for IEL) between time points as indicated; p -values are indicated, nd; not determined. Source data are provided as a Source Data file.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 7 MAIT cell-mediated protection in immune-compromised mice requires IFNgamma, TNF, and GM-CSF. A MAIT cell percentage of alphabeta-T cells in the liver and representative FACS plot showing intranuclear staining for T-bet and RORgammat of MAIT cells from donor C57BL/6 mice vaccinated with CpG and 5-OP-RU i.v. for 7 days, prior cell sorting for adoptive cell transfer. Pooled data from 7 (nil) or 11 (vaccinated) mice from 3 independent experiments (mean +- SEM). Unpaired t -test (two-tailed). P < 0.0001. B Schematic of protocol for MAIT cell adoptive transfer and F. tularensis LVS challenge: 10 5 liver MAIT cells from C57BL/6 (WT, shown in A ), Ifngamma -/- , Tnf -/- , Gm-csf -/- , or Il-17 -/- mice vaccinated with CpG (10 nmol) and 5-OP-RU (2 nmol) i.v. for 7 days were sorted by flow cytometry and transferred i.v. into Rag2 -/- gammaC -/- mice. The mice were treated with anti-CD4 and anti-CD8 mAb injection (i.p., 0.1 mg each) at days 1 and 3 post MAIT cell transfer, to deplete contaminating conventional T cells. After 2 weeks, mice were infected with an otherwise lethal dose (20 CFU) of F. tularensis LVS i.v. C Survival of untreated Rag2 -/- gammaC -/- mice or Rag2 -/- gammaC -/- mice following transfer of MAIT cells from WT, Ifngamma -/- , Tnf -/- , Gm-csf -/- , or Il-17 -/- mice according to schematic shown in B . Pooled data from two independent experiments with similar results ( n = 12-24 mice per group, as indicated). Log-rank tests ( Ifngamma -/- , Tnf -/- , Gm-csf -
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 Dynamics of Th1 cell population. A) Representative dot-plots of the population of CD4+Tbet+ cells of EAE animals. Dot-plots were obtained by previously gating in the CD3+ T cell population. Different quadrants were defined by application of the appropriate isotype control. A minimum of three animals per group was pooled and three replicates per score were analyzed. B and C) Histogrammes showing, respectively, the values corresponding to the total number and the percentage of CD4+Tbet+ cell population along EAE. Note that CD4+Tbet+ lymphocytes are found during the induction and peak phases and markedly decreased at score 2R of the recovery phase (ANOVA and Tukey's post-hoc test, *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
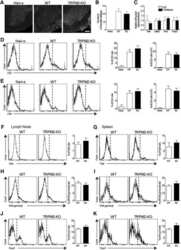
- Figure 1 Homozygous RhoA deletion in Treg cells leads to early, fatal spontaneous inflammatory disorders. (A) Survival outcome of RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/Flox Foxp3 YFP-Cre mice. Results were analyzed with a log-rank (Mantel-Cox) test and expressed as Kaplan-Meier survival curves. (B) Image of lymphadenopathy in RhoA Flox/Flox Foxp3 YFP-Cre mice. Inguinal lymph nodes are shown. (C) Images of H&E staining of the indicated organs from RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/Flox Foxp3 YFP-Cre mice (original magnification X 400). (D) Left, representative flow cytogram of CD44 and CD62L staining in CD4 + and CD8 + cells from the spleen of RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/Flox Foxp3 YFP-Cre mice. The numbers indicate percentages of CD44 + , CD44 + CD62L + , and CD62L + cells. Right, average percentages of CD44 + , CD44 + CD62L + , and CD62L + cells. (E) Left, representative flow cytogram of IL-17, IFN-gamma, and IL-4 staining in CD4 + Foxp3 - cells from the spleen of RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/Flox Foxp3 YFP-Cre mice. The numbers indicate percentages of IL-17 + , IFN-gamma + , and IL-4 + cells. Right, average percentages of IL-17 + , IFN-gamma + , and IL-4 + cells. (F) Left, representative flow cytogram of RORgammaT, T-bet and GATA3 staining in CD4 + Foxp3 - cells from the spleen of RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/Flox Foxp3 YFP-Cre mice. The numbers indicate percentages of RORgammaT + , T-bet + , and GATA3 + cells. Right, average percentages of RORgam
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
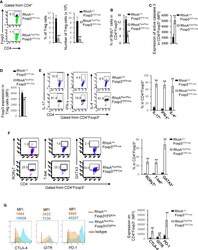
- Figure 2 Homozygous RhoA deletion in Treg cells dampens Treg cell homeostasis and induces Treg cell plasticity. (A) Left, representative flow cytogram of Foxp3 staining in CD4 + cells from the spleen of RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/Flox Foxp3 YFP-Cre mice. The numbers indicate percentages of CD4 + Foxp3 + Treg cells. Middle, average percentages of CD4 + Foxp3 + Treg cells. Right, average numbers of CD4 + Foxp3 + Treg cells. (B) Treg cell proliferation. Percentages of CD4 + Foxp3 + Treg cells incorporated with BrdU are shown. (C) Treg cell apoptosis. The expression levels (MFI: Mean fluorescence intensity) of active caspase 3 in CD4 + Foxp3 + Treg cells are shown. (D) The expression levels of Foxp3 in Treg cells. (E) Left, representative flow cytogram of IL-17, IFN-gamma, and IL-4 staining in CD4 + Foxp3 + Treg cells. The numbers indicate percentages of IL-17 + , IFN-gamma + , and IL-4 + Treg cells. Right, average percentages of IL-17 + , IFN-gamma + , and IL-4 + Treg cells. (F) Left, representative flow cytogram of RORgammaT, T-bet and GATA3 staining in CD4 + Foxp3 + Treg cells. The numbers indicate percentages of RORgammaT + , T-bet + , and GATA3 + Treg cells. Right, average percentages of RORgammaT + , T-bet + , and GATA3 + Treg cells. (G) Left, representative histogram of the expression levels of CTLA-4, GITR and PD-1 in CD4 + Foxp3 + Treg cells. The numbers above the graphs indicate MFI. Right, average MFI of CTLA-4, GITR and PD-1 in CD4 + Foxp3 + Treg cells. n =
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 Heterozygous RhoA deletion in Treg cells induces Treg cell plasticity and increases CD4 + effector T cells but does not result in autoimmunity. (A) Body weight of RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/+ Foxp3 YFP-Cre mice. (B) Images of H&E staining of the indicated organs. (C) Representative flow cytogram of Foxp3 staining in CD4 + cells from the spleen of RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/+ Foxp3 YFP-Cre mice. The numbers indicate percentages of CD4 + Foxp3 + Treg cells. (D) Left, average percentages of CD4 + Foxp3 + Treg cells. Right, cell numbers of CD4 + Foxp3 + Treg cells. (E) Proliferation of Foxp3 + YFP + Treg cells from RhoA +/+ Foxp3 YFP-Cre/+ and RhoA Flox/+ Foxp3 YFP-Cre/+ female mice. Percentages of Foxp3 + YFP + Treg cells incorporated with BrdU are shown. (F) Apoptosis of Foxp3 + YFP + Treg cells from RhoA +/+ Foxp3 YFP-Cre/+ and RhoA Flox/+ Foxp3 YFP-Cre/+ female mice. The expression levels (MFI: mean fluorescence intensity) of active caspase 3 in Foxp3 + YFP + Treg cells are shown. (G) The expression levels of Foxp3 in Treg cells from RhoA +/+ Foxp3 YFP-Cre and RhoA Flox/+ Foxp3 YFP-Cre mice. (H) Representative flow cytogram of IFN-gamma, IL-17 and IL-4 staining in CD4 + Foxp3 + Treg cells. The numbers indicate percentages of IFN-gamma + , IL-17 + and IL-4 + Treg cells. (I) Average percentages of IFN-gamma + , IL-17 + and IL-4 + Treg cells. (J) Representative flow cytogram of RORgammaT, T-bet and GATA3 staining in CD4 + Foxp3 + Treg cells. The numb
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 Effector CD4 T Cell Response to Adjuvanted Vaccines Groups of C57BL/6 mice were vaccinated IN, as in Figure 1 . At day 8 PV, cells from lungs and BAL were stained with I-A b /NP311 tetramers along with antibodies to cell surface molecules and transcription factors. (A) FACS plots show the percentages of I-A b /NP311 tetramer-binding cells among CD4 T cells. (B) Percentages of the indicated cell population among NP311-specific, tetramer-binding CD4 T cells. (C) FACS plots are gated on I-A b /NP311 tetramer-binding cells, and the numbers in each quadrant are the percentages of cells among the gated population; MFIs for transcription factors in NP311-specific CD4 T cells are plotted in the adjoining graphs. (D) FACS plots in (C) were used to quantify the percentages of T-bet LO EOMES HI cells (quadrant 4) among NP311-specific CD4 T cells. (E) Percentages of CD103 HI and CD69 HI cells among NP311-specific CD4 T cells. Data are representative of two independent experiments. Comparisons were made using one-way ANOVA test with Tukey-corrected multiple comparisons; *p < 0.05, **p < 0.01, and ***p < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 9 In vivo administration of dNP2-siChi3l1 complex inhibits pulmonary metastasis with enhanced Th1 and CTL effector molecules. a Experimental scheme of dNP2-siRNA complex treatment in pulmonary melanoma metastasis model. b Representative lung image of dNP2-siEGFP and dNP2-siChi3l1-treated mice. Scale bar, 2 mm c Number of pleural colonies in each lung was counted. Data are mean +- SEM of three sets of independent experiments and each dot in graphs represent an individual mouse. d Relative total tumor area in the lung was measured by Image J software 1.48 v. e Histology of lung sections by H&E staining, and infiltrated tumor region was measured by Image J software 1.48 v. Scale bar, 200 m f , g IFNgamma producing CD4 and CD8 T cells, and Granzyme B expression level in IFNgamma + CD8 T cells in the lung was analyzed by intracellular staining. % of IFNgamma + , and MFI of Granzyme B was represented as scattered graph. h Intracellular T-bet expression level in CD4 + IFNgamma + and CD8 + IFNgamma + population. MFI was represented as scattered graph. i mRNA expression of genes related to cytotoxicity and Th1 effector functions was analyzed by quantitative RT-PCR. Each gene expression level was normalized to beta-actin. Data are mean +- SD of three sets of independent experiments and each dot in graphs represent an individual mouse. n.s., not significant; * p < 0.05, ** p < 0.01, *** p < 0.001 (two-tailed Student's t -test)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2. Similarities between Infection-Induced Mouse FCRL5 + B Cells and atMBCs from Plasmodium -Infected Humans (A) RNA-seq was performed on class-switched (IgM - IgD - ), FCRL5 - or FCRL5 + B cells sorted from the blood of Ifngr1 - / - mice infected for 21 days with P. chabaudi . Columns represent individual mice (n = 5). Select genes involved in immune signaling are shown. Genes with putative activating function are labeled in green; those with putative inhibitory function are labeled in red. (B) Heatmaps depict all genes that are differentially regulated in both human atMBCs (left) and mouse FCRL5 + B cells, relative to cMBCs and FCRL5 - B cells, respectively. Human data are from . (C-E) Expression of (C) CD72 and CD38, (D) T-bet, and (E) CD11b, CD11c, CD86, and CD40 were measured by flow cytometry in blood B cells 21 d.p.i. **p < 0.01; ***p < 0.001 by paired t test (Wilcoxon rank-sum test). See also Figure S2 and Tables S1 and S2 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1. Characterization of CD4 + TIL, dLN, and Arm Transcriptomes by scRNA-Seq (A-D) TILs and dLN cells from wild-type (WT) mice at day 14 after MC38-GP injection analyzed by scRNA-seq and flow cytometry. (A) Heatmap shows row-standardized expression of selected genes across TIL and dLN clusters. Bar plot indicates the percentage of cells in each cluster relative to the total TIL or dLN cell number. (B) Flow cytometry contour plots of Foxp3 versus T-bet in CD44 hi GP66 + dLN cells (left) and in CD44 hi CD4 + splenocytes from tumor-free control mice (right). (C) Flow cytometry contour plots of Foxp3 versus T-bet in PD-1 + and GP66 + TILs (left) and in CD44 hi CD4 + splenocytes from tumor-free control mice (right). (B and C) Data representative from 18 tumor-bearing mice analyzed in four separate experiments. (D) t-SNE display of TILs and dLN cells, shaded gray by tissue origin (left) or color coded by main group (right, as defined in A). (E) t-SNE (TIL and dLN cell positioning as shown in B) display of normalized expression levels of selected genes. (F) Heatmap shows Pearson correlation between cluster fold change vectors (as defined in the text) across the two replicate experiments for TILs (left) and dLN cells (right). See also Figures S1 and S2 and Tables S1 and S6 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
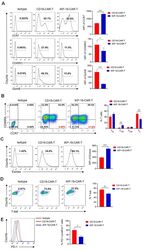
- Experimental details
- Figure 3 Characteristics of iKP-19-CAR-T cells and CD19-CAR-T cells. iKP-19-CAR-T cells or CD19-CAR-T cells were cultured for 10 days in X-VIVO media supplemented with 100 U/mL IL-2. ( A ) The expression of T cell differentiation markers in CAR-T cells was analyzed by flow cytometry using PE/Cy7-anti-human CCR7 antibody, PE-anti-human-CD45RO antibody and PE-anti-human GzmB antibody ( n = 4 different donors). ( B ) The frequency of naive (T N ; CCR7 + CD45RA + ), T CM (CCR7 + CD45RA - ), effector memory (T EM ; CCR7 - CD45RA - ) or effector (T E ; CCR7 - CD45RA + ) T cells were analyzed by flow cytometry using PE/Cy7-anti-human CCR7 antibody and FITC-anti-human CD45RA antibody ( n = 4 different donors). ( C ) The expression of transcription factor Eomes in CAR-T cells was analyzed by flow cytometry using FITC-anti-human Eomes antibody ( n = 4 different donors). ( D ) The expression of transcription factor T-bet in CAR-T cells was analyzed by flow cytometry using PE-anti-human T-bet antibody ( n = 4 different donors). ( E ) The expression of T cell exhaustion marker PD-1 in CAR-T cells was analyzed by flow cytometry using FITC-anti-human PD-1 antibody ( n = 4 different donors). All experiments were performed in triplicate manner using PBMCs from each donor and MFI or percentage was statistically analyzed. * p < 0.05, ** p < 0.01, *** p < 0.001. Error bars represent +- SD.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation