Antibody data
- Antibody Data
- Antigen structure
- References [42]
- Comments [0]
- Validations
- Immunocytochemistry [1]
- Immunohistochemistry [1]
- Other assay [21]
Submit
Validation data
Reference
Comment
Report error
- Product number
- A-6442 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Synapsin 1 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Purifed from natural sources
- Description
- This antibody has little or no activity against Synapsin II. Storage and reconstitution: when stored at -20ºC or below, the lyophilized antibody should retain full activity for over one year. Reconstitute using 50 µL PBS, pH 7.4, to yield a 0.2 mg/mL solution. If storing at 0-4ºC, add sodium azide to a final concentration of 2mM. For longer storage, divide the solution into aliquots and freeze at -20ºC, avoiding freeze/thaw cycles.
- Reactivity
- Human, Mouse, Rat, Bovine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 10 µg
- Concentration
- 0.2 mg/mL
- Storage
- -20°C
Submitted references Recombinant pro-CTSD (cathepsin D) enhances SNCA/α-Synuclein degradation in α-Synucleinopathy models.
Neurodegeneration and astrogliosis in the entorhinal cortex in Alzheimer's disease: Stereological layer-specific assessment and proteomic analysis.
Defective metabolic programming impairs early neuronal morphogenesis in neural cultures and an organoid model of Leigh syndrome.
Oncogenic UBE3C promotes breast cancer progression by activating Wnt/β-catenin signaling.
Neuronal MT1-MMP mediates ECM clearance and Lrp4 cleavage for agrin deposition and signaling in presynaptic development.
Decreased synthesis of ribosomal proteins in tauopathy revealed by non-canonical amino acid labelling.
Redundant and nonredundant organismal functions of EPS15 and EPS15L1.
Two-Dimensional Ti(3)C(2) MXene for High-Resolution Neural Interfaces.
Reversible Conformational Conversion of α-Synuclein into Toxic Assemblies by Glucosylceramide.
TDP-43 Depletion in Microglia Promotes Amyloid Clearance but Also Induces Synapse Loss.
Innervation of Cochlear Hair Cells by Human Induced Pluripotent Stem Cell-Derived Neurons In Vitro.
Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson's Patient Midbrain Neurons.
Rebuilding Brain Circuitry with Living Micro-Tissue Engineered Neural Networks.
A protocol for concurrent high-quality immunohistochemical and biochemical analyses in adult mouse central nervous system.
An in vitro model of developmental synaptogenesis using cocultures of human neural progenitors and cochlear explants.
Reelin immunoreactivity in neuritic varicosities in the human hippocampal formation of non-demented subjects and Alzheimer's disease patients.
α-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies.
Regional differentiation of retinoic acid-induced human pluripotent embryonic carcinoma stem cell neurons.
Synapse-to-neuron ratio is inversely related to neuronal density in mature neuronal cultures.
Proteomics analysis reveals overlapping functions of clustered protocadherins.
Chronic CXCL10 alters neuronal properties in rat hippocampal culture.
Three-dimensional neural constructs: a novel platform for neurophysiological investigation.
Cutting edge: MHC class I-Ly49 interaction regulates neuronal function.
Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo.
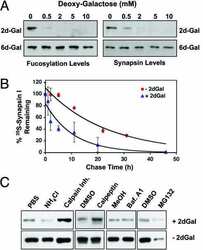
Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons.
Viral induction of central nervous system innate immune responses.
Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization.
Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein.
Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein.
Subtle neuronal death in striatum after short forebrain ischemia in rats detected by in situ end-labeling for DNA damage.
Synaptotagmin I is present mainly in autonomic and sensory neurons of the rat peripheral nervous system.
Development of the principal sensory nucleus of the trigeminal nerve of the rat and evidence for a transient synaptic field in the trigeminal sensory tract.
The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture.
The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture.
Cytosolic rat brain synapsin I is a diacylglycerol kinase.
Redistribution of synaptophysin and synapsin I during alpha-latrotoxin-induced release of neurotransmitter at the neuromuscular junction.
Developmental changes of synapsin I subcellular localization in rat cerebellar neurons.
The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1.
Synapsin I: an actin-bundling protein under phosphorylation control.
Presence of synapsin I in afferent and efferent nerve endings of vestibular sensory epithelia.
Characterization of synapsin I binding to small synaptic vesicles.
Synapsin I in nerve terminals: selective association with small synaptic vesicles.
Prieto Huarcaya S, Drobny A, Marques ARA, Di Spiezio A, Dobert JP, Balta D, Werner C, Rizo T, Gallwitz L, Bub S, Stojkovska I, Belur NR, Fogh J, Mazzulli JR, Xiang W, Fulzele A, Dejung M, Sauer M, Winner B, Rose-John S, Arnold P, Saftig P, Zunke F
Autophagy 2022 May;18(5):1127-1151
Autophagy 2022 May;18(5):1127-1151
Neurodegeneration and astrogliosis in the entorhinal cortex in Alzheimer's disease: Stereological layer-specific assessment and proteomic analysis.
Astillero-Lopez V, Gonzalez-Rodriguez M, Villar-Conde S, Flores-Cuadrado A, Martinez-Marcos A, Ubeda-Banon I, Saiz-Sanchez D
Alzheimer's & dementia : the journal of the Alzheimer's Association 2022 Dec;18(12):2468-2480
Alzheimer's & dementia : the journal of the Alzheimer's Association 2022 Dec;18(12):2468-2480
Defective metabolic programming impairs early neuronal morphogenesis in neural cultures and an organoid model of Leigh syndrome.
Inak G, Rybak-Wolf A, Lisowski P, Pentimalli TM, Jüttner R, Glažar P, Uppal K, Bottani E, Brunetti D, Secker C, Zink A, Meierhofer D, Henke MT, Dey M, Ciptasari U, Mlody B, Hahn T, Berruezo-Llacuna M, Karaiskos N, Di Virgilio M, Mayr JA, Wortmann SB, Priller J, Gotthardt M, Jones DP, Mayatepek E, Stenzel W, Diecke S, Kühn R, Wanker EE, Rajewsky N, Schuelke M, Prigione A
Nature communications 2021 Mar 26;12(1):1929
Nature communications 2021 Mar 26;12(1):1929
Oncogenic UBE3C promotes breast cancer progression by activating Wnt/β-catenin signaling.
Hang C, Zhao S, Wang T, Zhang Y
Cancer cell international 2021 Jan 6;21(1):25
Cancer cell international 2021 Jan 6;21(1):25
Neuronal MT1-MMP mediates ECM clearance and Lrp4 cleavage for agrin deposition and signaling in presynaptic development.
Oentaryo MJ, Tse AC, Lee CW
Journal of cell science 2020 Aug 5;133(15)
Journal of cell science 2020 Aug 5;133(15)
Decreased synthesis of ribosomal proteins in tauopathy revealed by non-canonical amino acid labelling.
Evans HT, Benetatos J, van Roijen M, Bodea LG, Götz J
The EMBO journal 2019 Jul 1;38(13):e101174
The EMBO journal 2019 Jul 1;38(13):e101174
Redundant and nonredundant organismal functions of EPS15 and EPS15L1.
Milesi C, Alberici P, Pozzi B, Oldani A, Beznoussenko GV, Raimondi A, Soppo BE, Amodio S, Caldieri G, Malabarba MG, Bertalot G, Confalonieri S, Parazzoli D, Mironov AA, Tacchetti C, Di Fiore PP, Sigismund S, Offenhäuser N
Life science alliance 2019 Feb;2(1)
Life science alliance 2019 Feb;2(1)
Two-Dimensional Ti(3)C(2) MXene for High-Resolution Neural Interfaces.
Driscoll N, Richardson AG, Maleski K, Anasori B, Adewole O, Lelyukh P, Escobedo L, Cullen DK, Lucas TH, Gogotsi Y, Vitale F
ACS nano 2018 Oct 23;12(10):10419-10429
ACS nano 2018 Oct 23;12(10):10419-10429
Reversible Conformational Conversion of α-Synuclein into Toxic Assemblies by Glucosylceramide.
Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H, Toker NJ, Jeon S, Fredriksen K, Mazzulli JR
Neuron 2018 Jan 3;97(1):92-107.e10
Neuron 2018 Jan 3;97(1):92-107.e10
TDP-43 Depletion in Microglia Promotes Amyloid Clearance but Also Induces Synapse Loss.
Paolicelli RC, Jawaid A, Henstridge CM, Valeri A, Merlini M, Robinson JL, Lee EB, Rose J, Appel S, Lee VM, Trojanowski JQ, Spires-Jones T, Schulz PE, Rajendran L
Neuron 2017 Jul 19;95(2):297-308.e6
Neuron 2017 Jul 19;95(2):297-308.e6
Innervation of Cochlear Hair Cells by Human Induced Pluripotent Stem Cell-Derived Neurons In Vitro.
Gunewardene N, Crombie D, Dottori M, Nayagam BA
Stem cells international 2016;2016:1781202
Stem cells international 2016;2016:1781202
Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson's Patient Midbrain Neurons.
Mazzulli JR, Zunke F, Tsunemi T, Toker NJ, Jeon S, Burbulla LF, Patnaik S, Sidransky E, Marugan JJ, Sue CM, Krainc D
The Journal of neuroscience : the official journal of the Society for Neuroscience 2016 Jul 20;36(29):7693-706
The Journal of neuroscience : the official journal of the Society for Neuroscience 2016 Jul 20;36(29):7693-706
Rebuilding Brain Circuitry with Living Micro-Tissue Engineered Neural Networks.
Struzyna LA, Wolf JA, Mietus CJ, Adewole DO, Chen HI, Smith DH, Cullen DK
Tissue engineering. Part A 2015 Nov;21(21-22):2744-56
Tissue engineering. Part A 2015 Nov;21(21-22):2744-56
A protocol for concurrent high-quality immunohistochemical and biochemical analyses in adult mouse central nervous system.
Notter T, Panzanelli P, Pfister S, Mircsof D, Fritschy JM
The European journal of neuroscience 2014 Jan;39(2):165-75
The European journal of neuroscience 2014 Jan;39(2):165-75
An in vitro model of developmental synaptogenesis using cocultures of human neural progenitors and cochlear explants.
Nayagam BA, Edge AS, Needham K, Hyakumura T, Leung J, Nayagam DA, Dottori M
Stem cells and development 2013 Mar 15;22(6):901-12
Stem cells and development 2013 Mar 15;22(6):901-12
Reelin immunoreactivity in neuritic varicosities in the human hippocampal formation of non-demented subjects and Alzheimer's disease patients.
Notter T, Knuesel I
Acta neuropathologica communications 2013 Jun 26;1:27
Acta neuropathologica communications 2013 Jun 26;1:27
α-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies.
Lim Y, Kehm VM, Lee EB, Soper JH, Li C, Trojanowski JQ, Lee VM
The Journal of neuroscience : the official journal of the Society for Neuroscience 2011 Jul 6;31(27):10076-87
The Journal of neuroscience : the official journal of the Society for Neuroscience 2011 Jul 6;31(27):10076-87
Regional differentiation of retinoic acid-induced human pluripotent embryonic carcinoma stem cell neurons.
Coyle DE, Li J, Baccei M
PloS one 2011 Jan 20;6(1):e16174
PloS one 2011 Jan 20;6(1):e16174
Synapse-to-neuron ratio is inversely related to neuronal density in mature neuronal cultures.
Cullen DK, Gilroy ME, Irons HR, Laplaca MC
Brain research 2010 Nov 4;1359:44-55
Brain research 2010 Nov 4;1359:44-55
Proteomics analysis reveals overlapping functions of clustered protocadherins.
Han MH, Lin C, Meng S, Wang X
Molecular & cellular proteomics : MCP 2010 Jan;9(1):71-83
Molecular & cellular proteomics : MCP 2010 Jan;9(1):71-83
Chronic CXCL10 alters neuronal properties in rat hippocampal culture.
Cho J, Nelson TE, Bajova H, Gruol DL
Journal of neuroimmunology 2009 Feb 15;207(1-2):92-100
Journal of neuroimmunology 2009 Feb 15;207(1-2):92-100
Three-dimensional neural constructs: a novel platform for neurophysiological investigation.
Irons HR, Cullen DK, Shapiro NP, Lambert NA, Lee RH, Laplaca MC
Journal of neural engineering 2008 Sep;5(3):333-41
Journal of neural engineering 2008 Sep;5(3):333-41
Cutting edge: MHC class I-Ly49 interaction regulates neuronal function.
Zohar O, Reiter Y, Bennink JR, Lev A, Cavallaro S, Paratore S, Pick CG, Brooker G, Yewdell JW
Journal of immunology (Baltimore, Md. : 1950) 2008 May 15;180(10):6447-51
Journal of immunology (Baltimore, Md. : 1950) 2008 May 15;180(10):6447-51
Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo.
Vereyken EJ, Bajova H, Chow S, de Graan PN, Gruol DL
The European journal of neuroscience 2007 Jun;25(12):3605-16
The European journal of neuroscience 2007 Jun;25(12):3605-16
Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons.
Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B, Hsieh-Wilson LC
Proceedings of the National Academy of Sciences of the United States of America 2006 Jan 3;103(1):21-6
Proceedings of the National Academy of Sciences of the United States of America 2006 Jan 3;103(1):21-6
Viral induction of central nervous system innate immune responses.
Rempel JD, Quina LA, Blakely-Gonzales PK, Buchmeier MJ, Gruol DL
Journal of virology 2005 Apr;79(7):4369-81
Journal of virology 2005 Apr;79(7):4369-81
Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization.
Paradis S, Sweeney ST, Davis GW
Neuron 2001 Jun;30(3):737-49
Neuron 2001 Jun;30(3):737-49
Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein.
Galvin JE, Uryu K, Lee VM, Trojanowski JQ
Proceedings of the National Academy of Sciences of the United States of America 1999 Nov 9;96(23):13450-5
Proceedings of the National Academy of Sciences of the United States of America 1999 Nov 9;96(23):13450-5
Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein.
Galvin JE, Uryu K, Lee VM, Trojanowski JQ
Proceedings of the National Academy of Sciences of the United States of America 1999 Nov 9;96(23):13450-5
Proceedings of the National Academy of Sciences of the United States of America 1999 Nov 9;96(23):13450-5
Subtle neuronal death in striatum after short forebrain ischemia in rats detected by in situ end-labeling for DNA damage.
Schmidt-Kastner R, Fliss H, Hakim AM
Stroke 1997 Jan;28(1):163-9; discussion 169-70
Stroke 1997 Jan;28(1):163-9; discussion 169-70
Synaptotagmin I is present mainly in autonomic and sensory neurons of the rat peripheral nervous system.
Li JY, Jahn R, Dahlström A
Neuroscience 1994 Dec;63(3):837-50
Neuroscience 1994 Dec;63(3):837-50
Development of the principal sensory nucleus of the trigeminal nerve of the rat and evidence for a transient synaptic field in the trigeminal sensory tract.
al-Ghoul WM, Miller MW
The Journal of comparative neurology 1993 Apr 22;330(4):476-90
The Journal of comparative neurology 1993 Apr 22;330(4):476-90
The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture.
Fletcher TL, Cameron P, De Camilli P, Banker G
The Journal of neuroscience : the official journal of the Society for Neuroscience 1991 Jun;11(6):1617-26
The Journal of neuroscience : the official journal of the Society for Neuroscience 1991 Jun;11(6):1617-26
The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture.
Fletcher TL, Cameron P, De Camilli P, Banker G
The Journal of neuroscience : the official journal of the Society for Neuroscience 1991 Jun;11(6):1617-26
The Journal of neuroscience : the official journal of the Society for Neuroscience 1991 Jun;11(6):1617-26
Cytosolic rat brain synapsin I is a diacylglycerol kinase.
Kahn DW, Besterman JM
Proceedings of the National Academy of Sciences of the United States of America 1991 Jul 15;88(14):6137-41
Proceedings of the National Academy of Sciences of the United States of America 1991 Jul 15;88(14):6137-41
Redistribution of synaptophysin and synapsin I during alpha-latrotoxin-induced release of neurotransmitter at the neuromuscular junction.
Torri-Tarelli F, Villa A, Valtorta F, De Camilli P, Greengard P, Ceccarelli B
The Journal of cell biology 1990 Feb;110(2):449-59
The Journal of cell biology 1990 Feb;110(2):449-59
Developmental changes of synapsin I subcellular localization in rat cerebellar neurons.
Harada A, Sobue K, Hirokawa N
Cell structure and function 1990 Dec;15(6):329-42
Cell structure and function 1990 Dec;15(6):329-42
The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1.
Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H
The Journal of cell biology 1989 Jan;108(1):111-26
The Journal of cell biology 1989 Jan;108(1):111-26
Synapsin I: an actin-bundling protein under phosphorylation control.
Petrucci TC, Morrow JS
The Journal of cell biology 1987 Sep;105(3):1355-63
The Journal of cell biology 1987 Sep;105(3):1355-63
Presence of synapsin I in afferent and efferent nerve endings of vestibular sensory epithelia.
Favre D, Scarfone E, Di Gioia G, De Camilli P, Dememes D
Brain research 1986 Oct 8;384(2):379-82
Brain research 1986 Oct 8;384(2):379-82
Characterization of synapsin I binding to small synaptic vesicles.
Schiebler W, Jahn R, Doucet JP, Rothlein J, Greengard P
The Journal of biological chemistry 1986 Jun 25;261(18):8383-90
The Journal of biological chemistry 1986 Jun 25;261(18):8383-90
Synapsin I in nerve terminals: selective association with small synaptic vesicles.
Navone F, Greengard P, De Camilli P
Science (New York, N.Y.) 1984 Dec 7;226(4679):1209-11
Science (New York, N.Y.) 1984 Dec 7;226(4679):1209-11
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
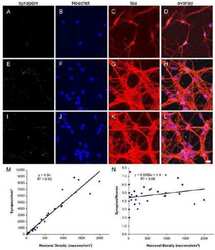
- Immunofluorescent analysis of PSD-95 (green) and MAP2 (red) on rat primary cortical neurons cultured for 28 days in the B-27 Plus Neuronal Culture System (Product # A3653401). At day 28 the cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% triton x-100 for 30min, and blocked with 1% BSA for 30 min at room temperature. Cells were stained with anti-Synapsin-1 antibody (Product # A-6442) at a dilution of 1:500, and anti-MAP2 (Product # 13-1500) at a dilution of 1:250, in 1% BSA staining buffer, overnight at 4C, and then incubated with Alexa Fluor 488 conjugated donkey anti-rabbit (Product # A-21206) and Alexa Fluor 594 donkey anti-mouse (Product # A-21203) antibodies at a dilution of 1:1000 for 30 min. at room temp. Wash 3 times with DPBS. Stain with DAPI for nucleus. Images were taken on a Thermo Fisher Scientific EVOS M5000 Cell Imaging System at 10x magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Peripheral neurons in mouse intestinal cryosections were labeled with rabbit anti-synapsin I antibody (Product # A-6442) and detected using Alexa Fluor® 488 goat anti-rabbit IgG antibody (Product # A-11008). This tissue was counterstained with DAPI (Product # D1306, D3571, D21490).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
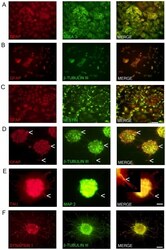
- Figure 1 Marker expression of NTERA2 (NT2) cells and ATRA differentiated NTERA2 neurons (NT2N). NT2 cells (A-C) co-express GFAP and SSEA3 with GFAP staining confined to the nucleus (A). NT2 cells also co-express GFAP and beta-tubulin III (BT3) which are both mainly confined to the nucleus (B). Nestin with GFAP are co-localized in NT2 cells (C). NT2N assembled themselves into cell clusters which co-expressed GFAP and BT3 found within the cytoplasm and neurites (D). Arrow heads point to cells that appear as radial glia cells which only display GFAP staining (D). NT2N also express neural markers TAU and MAP2 with MAP2 confined to dendrites (tapering morphology shown by arrow heads) and TAU not displaying restriction to axons. In merge panel insert shows close-up view of neurite (E). Synapsin 1 staining is seen as punctate along neurite projections co-expressing BT3 (F). Photomicrographs were obtained at 100X (A-C; scale bar 200 um) and 200X (D-F; scale bar 100 um).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
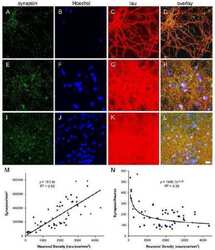
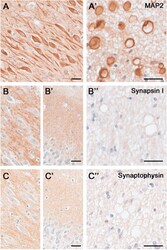
- Figure 5 Dendritic and synaptic proteins in the aged human hippocampus: Differential association with CAm. Immunoperoxidase-hematoxylin staining of brain sections obtained from an 85 year-old ND individual. A) Representative images of the CA2 subfield and fornix (A') labeled with the dendritic cytoskeletal marker anti-MAP2. Note the strong immunoreactivity in pyramidal cell bodies and dendrites, as well as CAm (A') . B- C) Immunoperoxidase staining using anti-Synapsin-1 (B) and anti-Synaptophysin antibodies (C) depicting the CA2 pyramidal cell layer (B, C) , the dentate gyrus molecular layer (B', C') and the fornix (B"", C"") . While a characteristic synaptic immunoreactivity of both markers was evident in the hippocampus proper and dentate gyrus, no accumulation of these synaptic proteins was seen in CAm. Scale bars: A- C = 30 mum, A'- C' = 30 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
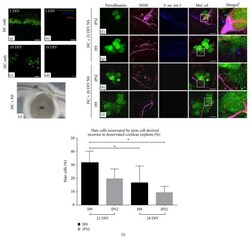
- Figure 4 The synaptic potential of 21- and 28-day-old hiPSC- and hESC-derived neurons. (a-b) In the denervated cochlear explant controls at 1 DIV, some accumulation of residual NFM within hair cell somata and a few synaptic puncta were observed. (c-d) After 10 DIV, there appeared to be fewer hair cells with NFM accumulation and synapsin 1 was undetectable. (e) Light microscope image of denervated cochlear explant cocultured with stem cell-derived neurosphere. (f and g) The hiPSC and hESC neurospheres cocultured at 21 DIV extended their neural processes towards hair cells in the denervated explants. Punctate-like synapsin 1 expression was observed along the neural processes of both the hiPSC- and hESC-derived neurites making contact with hair cells. (h and i) The hiPSC and hESC neurospheres cocultured at 28 DIV projected fewer neural processes towards hair cells in the denervated explants. Synapsin 1 was rarely observed along the neural processes of both the hiPSC- and hESC-derived neurites making contact with hair cells. The merged 1 images represent higher magnification images of the boxed inserts and depict the punctate-like synaptic contacts made between the stem cell-derived neural processes and hair cells. Scale bar = 20 mu m (applicable for all images). (j) The 21-day-old hiPSC- and hESC-derived neurons made contact with a significantly higher number of hair cells, compared to the 28-day-old neurons (H9: p < 0.05). Furthermore, the 21-day-old hESC neurons made contact w
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
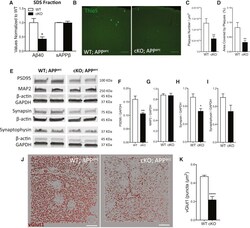
- Figure 3 Depletion of TDP-43 from Microglia Enhances Amyloid Clearance but Exacerbates Synaptic Loss in a Mouse Model of AD (A) Multiplexed electrocheminoluminescent assay measurements of Abeta40 and sAPPbeta levels in the SDS-soluble fraction of cortex homogenates from 7-month-old APP arc mice lacking TDP-43 in microglia. Mean +- SEM, n = 4 mice per genotype, ** p < 0.01, using two-way ANOVA, followed by Sidak's post hoc test. (B) Representative max-projections of confocal stacks from cortex of WT;APP arc or cKO;APP arc mice stained with Thioflavin S. (C and D) Quantification of ThioS plaque density (C) and area covered by plaques from the cortex of 7-month-old APP arc , WT (n = 36) and cKO (n = 36) with acquisitions from 4 animals per genotype (D). Mean +- SEM, ** p < 0.01, using two-tailed unpaired t test. (E-I) Representative blots for synaptic markers, from the cortex of 7-month-old APP arc mice, WT or KO for microglial TDP-43 (E). Quantification of western blots for PSD95 (F), MAP2 (G), synapsin (H), and synaptophysin (I) normalized for GAPDH reference gene. Mean +- SEM, n = 4-5 mice per genotype, * p < 0.05, ** p < 0.01, using two-tailed unpaired t test. (J and K), Representative 3D reconstruction from confocal acquisitions of vGlut1 immunoreactivity in the cortex of WT and cKO mice (J) and relative quantification (K) (WT n = 25, cKO n = 17, acquisitions from 4 mice per genotype; **** p < 0.0001, two-tailed t test).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Comparative immunoreactivity of a selected panel of antibodies in perfusion-fixed and immersion-fixed tissue. Immersion fixation was carried out immediately following perfusion with ACSF (see ). Images are from immunoperoxidase staining and photographed in bright-field (A-F; G', H', I', J') or dark-field (G, H, I, J) transmission microscopy. Each staining pattern is illustrated at two different magnifications to highlight the regional and cellular distribution of the markers investigated. The regions selected include the dorsal hippocampus (A-F; I-J) and the occipital cortex (G-H). (A, B) CD 68 is a microglial cell marker, expressed at low levels in resting microglia; as seen in A' and B', both fixation methods are compatible with the staining of fine ramified processes of resting microglia. (C, D) GFAP is a marker of astrocytes, strongly expressed in the hippocampal formation, depicted in tissue from old mice (16 and 19 months) to illustrate that immersion-fixation is also suitable for tissue from aged mice. (E, F) S ynapsin 1 is a presynaptic protein, strongly expressed in mossy fibers innervating CA 3 pyramidal cells; the two fixation protocols provide similar results, with a clear distinction of these terminal in the stratum lucidum (sl) and a weaker staining in the stratum pyramidale (sp) and radiatum (sr). (G, H) Distribution of serotonergic (5- HT ) axons in the neocortex, depicted in both bright- and dark-field to illustrate that a similar signal-to-noise ratio and mo
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 2 The silencing efficiency of UBE3C in BrCa cells. a , b The silencing efficiency of UBE3C in BrCa cells was confirmed by qPCR and western blotting analysis. SiRNA #2 and siRNA #1 showed highest silencing efficiency for UBE3C in MCF-7 and MDA-MB-453 cells, respectively. ***P < 0.001. c , d The silencing efficiency of UBE3C in BrCa cells and cytomorphology was determined by microscopy and immunofluorescence analysis. Green fluorescence indicated that the cells have been successfully transfected. ***P < 0.001. Bar = 200 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 4 The efficiency of UBE3C overexpression in BrCa cells. a , b The efficiency of UBE3C overexpression in BrCa cells was confirmed by qPCR and western blotting analysis. UBE3C-overexpressing vector showed satisfactory transfection efficiency. c , d The efficiency of UBE3C overexpression in BrCa cells and cytomorphology was determined by microscopy and immunofluorescence analysis. Green fluorescence indicated that the cells have been successfully transfected. ***P < 0.001. Bar = 200 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 6 UBE3C promotes the nuclear accumulation of beta-catenin. a , b Immunofluorescence staining shows that silencing or overexpressing UBE3C in BrCa cells inhibited or increased the nuclear accumulation of beta-catenin, respectively. Blue: DAPI, green: beta-catenin, red: UBE3C. Bar = 20 mum. c , d The expression levels of total and nuclear beta-catenin in UBE3C-silenced and/or UBE3C-overexpressing BrCa cells was evaluated by western blotting. UBE3C-silenced BrCa cells showed low nuclear beta-catenin level, but UBE3C-overexpressing BrCa cells were opposite. **P < 0.01, ***P < 0.001
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 7. Intraneuronal effect of rHsCTSD on SNCA and synaptical vesicles in primary neurons and in DA-iPSn. (A) Representative immunostaining of neuronal processes of primary neurons derived from WT, ctsd KO and ctsd KO +rHsCTSD animals. SNCA shown by antibody anti-syn-1 in red and SYN-1 (synapsin I) in green. Scale bar: 5 um. (B) Quantification of SYN-1-positive vesicle cluster number per um and (C) of the average SYN-1-positive vesicle size per punctum (um) in primary neurons from WT, ctsd KO and ctsd KO +rHsCTSD (analysis of n = 11-13 individual neurons per group). (D) Representative immunostaining of SNCA (LB509; red) and SYN-1 (green) in neuronal processes of DA-iPSn of isogenic control (A53T corr) and mutant line (A53T) with and without rHsCTSD treatment. Scale bar: 5 um. (E) Quantification of SYN-1-positive vesicle cluster numbers per um and (F) analyses of average SYN-1-positive vesicle size per punctum (um) in neuronal processes of DA-iPSn A53T corr. +PBS, A53T +PBS and A53T +rHsCTSD (n = 13-16 individual neurons per group). (G and H) Representative structured illumination microscopy (SIM) images providing detailed localization of SNCA respective to SYN-1 signal in (G) primary neurons derived from KO +PBS and KO +rHsCTSD animals and (H) DA-iPSn from A53T mutant +PBS and +rHsCTSD. Neuronal processes were immunostained with SNCA (Antibody: syn-1 for primary neurons and LB509 for DA-iPSn; magenta) and SYN-1 (green). Scale bar: overview 2 um and inset 1 um. Images highl
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry