MA5-17040
antibody from Invitrogen Antibodies
Targeting: CCL2
GDCF-2, HC11, MCAF, MCP-1, MCP1, MGC9434, SCYA2, SMC-CF
Antibody data
- Antibody Data
- Antigen structure
- References [15]
- Comments [0]
- Validations
- Western blot [3]
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Flow cytometry [1]
- Other assay [14]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA5-17040 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- MCP-1 Monoclonal Antibody (2D8)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA5-17040 targets CCL2 in indirect ELISA, FACS, ICC, IHC, IF and WB applications and shows reactivity with Human, Mouse, and Non-human primate samples. The MA5-17040 immunogen is purified recombinant fragment of human CCL2 expressed in E. Coli. MA5-17040 detects CCL2 which has a predicted molecular weight of approximately 11kDa.
- Reactivity
- Human, Mouse
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 2D8
- Vial size
- 100 µg
- Concentration
- 1 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Environment-Sensitive Ectodomain Shedding of Epithin/PRSS14 Increases Metastatic Potential of Breast Cancer Cells by Producing CCL2.
Epstein-Barr viral product-containing exosomes promote fibrosis and nasopharyngeal carcinoma progression through activation of YAP1/FAPα signaling in fibroblasts.
IL-15 Prevents Renal Fibrosis by Inhibiting Collagen Synthesis: A New Pathway in Chronic Kidney Disease?
Therapeutic Benefit of Galectin-1: Beyond Membrane Repair, a Multifaceted Approach to LGMD2B.
Impact of the rs1024611 Polymorphism of CCL2 on the Pathophysiology and Outcome of Primary Myelofibrosis.
Tart Cherry Juice and Seeds Affect Pro-Inflammatory Markers in Visceral Adipose Tissue of High-Fat Diet Obese Rats.
Assessment of Immunological Potential of Glial Restricted Progenitor Graft In Vivo-Is Immunosuppression Mandatory?
Wnt5a promotes renal tubular inflammation in diabetic nephropathy by binding to CD146 through noncanonical Wnt signaling.
Epicardial placement of human MSC-loaded fibrin sealant films for heart failure: Preclinical efficacy and mechanistic data.
Knockdown of Leptin Receptor Affects Macrophage Phenotype in the Tumor Microenvironment Inhibiting Breast Cancer Growth and Progression.
The Polycomb Repressor Complex 1 Drives Double-Negative Prostate Cancer Metastasis by Coordinating Stemness and Immune Suppression.
Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress.
Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages.
Liver macrophage-associated inflammation correlates with SIV burden and is substantially reduced following cART.
Diminished bone regeneration after debridement of posttraumatic osteomyelitis is accompanied by altered cytokine levels, elevated B cell activity, and increased osteoclast activity.
Jang J, Cho EH, Cho Y, Ganzorig B, Kim KY, Kim MG, Kim C
Molecules and cells 2022 Aug 31;45(8):564-574
Molecules and cells 2022 Aug 31;45(8):564-574
Epstein-Barr viral product-containing exosomes promote fibrosis and nasopharyngeal carcinoma progression through activation of YAP1/FAPα signaling in fibroblasts.
Lee PJ, Sui YH, Liu TT, Tsang NM, Huang CH, Lin TY, Chang KP, Liu SC
Journal of experimental & clinical cancer research : CR 2022 Aug 20;41(1):254
Journal of experimental & clinical cancer research : CR 2022 Aug 20;41(1):254
IL-15 Prevents Renal Fibrosis by Inhibiting Collagen Synthesis: A New Pathway in Chronic Kidney Disease?
Devocelle A, Lecru L, Ferlicot S, Bessede T, Candelier JJ, Giron-Michel J, François H
International journal of molecular sciences 2021 Oct 28;22(21)
International journal of molecular sciences 2021 Oct 28;22(21)
Therapeutic Benefit of Galectin-1: Beyond Membrane Repair, a Multifaceted Approach to LGMD2B.
Vallecillo-Zúniga ML, Poulson PD, Luddington JS, Arnold CJ, Rathgeber M, Kartchner BC, Hayes S, Gill H, Valdoz JC, Spallino JL, Garfield S, Dodson EL, Arthur CM, Stowell SR, Van Ry PM
Cells 2021 Nov 17;10(11)
Cells 2021 Nov 17;10(11)
Impact of the rs1024611 Polymorphism of CCL2 on the Pathophysiology and Outcome of Primary Myelofibrosis.
Masselli E, Carubbi C, Pozzi G, Percesepe A, Campanelli R, Villani L, Gobbi G, Bonomini S, Roti G, Rosti V, Massa M, Barosi G, Vitale M
Cancers 2021 May 22;13(11)
Cancers 2021 May 22;13(11)
Tart Cherry Juice and Seeds Affect Pro-Inflammatory Markers in Visceral Adipose Tissue of High-Fat Diet Obese Rats.
Moruzzi M, Klöting N, Blüher M, Martinelli I, Tayebati SK, Gabrielli MG, Roy P, Micioni Di Bonaventura MV, Cifani C, Lupidi G, Amenta F, Tomassoni D
Molecules (Basel, Switzerland) 2021 Mar 5;26(5)
Molecules (Basel, Switzerland) 2021 Mar 5;26(5)
Assessment of Immunological Potential of Glial Restricted Progenitor Graft In Vivo-Is Immunosuppression Mandatory?
Kozlowska U, Klimczak A, Bednarowicz KA, Zalewski T, Rozwadowska N, Chojnacka K, Jurga S, Barnea ER, Kurpisz MK
Cells 2021 Jul 16;10(7)
Cells 2021 Jul 16;10(7)
Wnt5a promotes renal tubular inflammation in diabetic nephropathy by binding to CD146 through noncanonical Wnt signaling.
Li X, Wen J, Dong Y, Zhang Q, Guan J, Liu F, Zhou T, Li Z, Fan Y, Wang N
Cell death & disease 2021 Jan 18;12(1):92
Cell death & disease 2021 Jan 18;12(1):92
Epicardial placement of human MSC-loaded fibrin sealant films for heart failure: Preclinical efficacy and mechanistic data.
Fields L, Ito T, Kobayashi K, Ichihara Y, Podaru MN, Hussain M, Yamashita K, Machado V, Lewis-McDougall F, Suzuki K
Molecular therapy : the journal of the American Society of Gene Therapy 2021 Aug 4;29(8):2554-2570
Molecular therapy : the journal of the American Society of Gene Therapy 2021 Aug 4;29(8):2554-2570
Knockdown of Leptin Receptor Affects Macrophage Phenotype in the Tumor Microenvironment Inhibiting Breast Cancer Growth and Progression.
Gelsomino L, Naimo GD, Malivindi R, Augimeri G, Panza S, Giordano C, Barone I, Bonofiglio D, Mauro L, Catalano S, Andò S
Cancers 2020 Jul 27;12(8)
Cancers 2020 Jul 27;12(8)
The Polycomb Repressor Complex 1 Drives Double-Negative Prostate Cancer Metastasis by Coordinating Stemness and Immune Suppression.
Su W, Han HH, Wang Y, Zhang B, Zhou B, Cheng Y, Rumandla A, Gurrapu S, Chakraborty G, Su J, Yang G, Liang X, Wang G, Rosen N, Scher HI, Ouerfelli O, Giancotti FG
Cancer cell 2019 Aug 12;36(2):139-155.e10
Cancer cell 2019 Aug 12;36(2):139-155.e10
Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress.
Li DJ, Fu H, Tong J, Li YH, Qu LF, Wang P, Shen FM
Redox biology 2018 May;15:22-33
Redox biology 2018 May;15:22-33
Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages.
Rhys HI, Dell'Accio F, Pitzalis C, Moore A, Norling LV, Perretti M
EBioMedicine 2018 Mar;29:60-69
EBioMedicine 2018 Mar;29:60-69
Liver macrophage-associated inflammation correlates with SIV burden and is substantially reduced following cART.
Fisher BS, Green RR, Brown RR, Wood MP, Hensley-McBain T, Fisher C, Chang J, Miller AD, Bosche WJ, Lifson JD, Mavigner M, Miller CJ, Gale M Jr, Silvestri G, Chahroudi A, Klatt NR, Sodora DL
PLoS pathogens 2018 Feb;14(2):e1006871
PLoS pathogens 2018 Feb;14(2):e1006871
Diminished bone regeneration after debridement of posttraumatic osteomyelitis is accompanied by altered cytokine levels, elevated B cell activity, and increased osteoclast activity.
Wagner JM, Jaurich H, Wallner C, Abraham S, Becerikli M, Dadras M, Harati K, Duhan V, Khairnar V, Lehnhardt M, Behr B
Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2017 Nov;35(11):2425-2434
Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2017 Nov;35(11):2425-2434
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of CCL2 using a CCL2 monoclonal antibody (Product # MA5-17040) against a human CCL2 (AA: 1-99) recombinant protein.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of CCL2 using a CCL2 monoclonal antibody (Product # MA5-17040) against a human CCL2 (AA: 1-99) recombinant protein.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of CCL2 using CCL2 monoclonal antibody (Product # MA5-17040) in A549 (1), HeLa (2), Raw264.7 (3), L1210 (4), C6 (5), and COS-7 (6)cell lysate.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescence analysis of HepG2 cells using CCL2 monoclonal antibody (Product # MA5-17040) (Green). Blue: DRAQ5 fluorescent DNA dye. Red: actin filaments have been labeled with phalloidin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescence analysis of HepG2 cells using CCL2 monoclonal antibody (Product # MA5-17040) (Green). Blue: DRAQ5 fluorescent DNA dye. Red: actin filaments have been labeled with phalloidin.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemical analysis of paraffin-embedded liver cancer tissues using CCL2 monoclonal antibody (Product # MA5-17040) followed with DAB staining.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Flow cytometric analysis of A549 cells using CCL2 monoclonal antibody (Product # MA5-17040) (green) and negative control (red).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
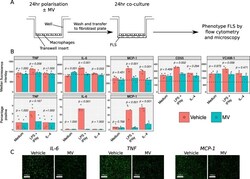
- Experimental details
- Fig. 5 Effect of neutrophil MVs on macrophage-FLS co-culture. A) Experiment scheme. Monocyte-derived macrophages were treated with 10 ng/mL LPS and 20 ng/mL IFN-gamma, 50 ng/mL IL-4 or vehicle for 24 h, in the presence of 3 x 10 6 MV/mL or vehicle. Macrophages were washed and co-cultured with FLS for a further 24 h, after which time FLS were immunophenotyped by flow cytometry. The experiment was repeated with 3 different macrophage donors and the mean across the donors was taken for each replicate. B) Antigen expression data for each FLS donor (mean expression across co-culture with 3 different macrophage donors) where dotted lines indicate median fluorescence of isotype-matched control samples. C) Confirmatory immunofluorescence images of IL-6, TNF-alpha and MCP-1 expression after co-culture with macrophages after classical activation, in the presence of 3 x 10 6 MV/mL or vehicle. Data in B analysed with separate two-way ANOVA with Holm-Sidak post-hoc tests for each antigen. Fig. 5
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 CCL2 expression by PMF MNCs according to the rs1024611 SNP. Fold-changes (2-[(CT CCL2-CT GAPDH)IL1-beta - (CT CCL2-CT GAPDH)untr, relative 2-DeltaDeltaCT values) in CCL2 mRNA expression upon ex vivo IL1-beta stimulation of MNCs from PMF patients stratified according to the rs1024611 genotype: A/A (n. 13) vs. A/G (n. 7) vs. G/G (n.5) ( A ) and A/A + A/G (n. 20) vs. G/G (n. 5) ( B ). Data are expressed as mean +- SEM. * p < 0.05, ** p < 0.01 by one-way ANOVA followed by Tukey test ( A ) or t -test ( B ). Fold-changes [(O.D.CCL2/O.D.GAPDH)IL-1beta/[(O.D.CCL2/O.D.GAPDH)UNTR, relative western blot optical density (O.D.) values] in CCL2 protein expression upon ex vivo IL1-beta stimulation of MNCs from PMF patients stratified according to the rs1024611 genotype A/A, n. 6 vs. A/G, n. 7 vs. G/G, n.5 ( C ) and A/A + A/G, n. 13 vs. G/G n. 5 ( E ). Data are expressed as mean +- SEM. ** p < 0.01, *** p < 0.001 by ANOVA followed by Tukey test ( C ) or t -test ( E ). ( D ) Western blot showing CCL2 protein expression in MNCs from a representative A/A, A/G and G/G PMF, at a basal state (-) and upon IL1-beta stimulation (+).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 Effects of ruxolitinib on CCL2 and CCR2 expression ( A ) Fold-decrease curves (2-[(CT CCL2-CT GAPDH)IL1-beta - (CT CCL2-CT GAPDH)untr, relative 2-DeltaDeltaCT values) of CCL2 mRNA expression upon ex vivo IL1-beta stimulation of MNCs from 4 PMF patients before (T0) and after 1 month (T1) of ruxolitinib therapy. T0 values were set as reference. ( B ) Fold-decrease (2-[(CT CCL2-CT GAPDH)IL1-beta - (CT CCL2-CT GAPDH)untr, relative 2-DeltaDeltaCT values) of CCL2 mRNA expression upon ex vivo IL1-beta stimulation of MNCs from 4 PMF patients before (T0) and after 1 month (T1) of ruxolitinib therapy. Data are expressed ad mean +- SEM as compared to T0 (*** p < 0.001 by t -test). ( C ) Fold-decrease curves [(O.D.CCL2/O.D.GAPDH)IL-1beta/[(O.D.CCL2/O.D.GAPDH)UNTR, relative western blot optical density (O.D.) values] of CCL2 protein expression upon ex vivo IL1-beta stimulation of MNCs from 3 PMF patients before (T0) and after 1 (T1) and 3 months (T2) of ruxolitinib therapy. T0 values are set as reference. ( D ) Representative Western blot showing CCL2 protein expression in MNCs from the PMF#20.6, at a basal state (-) and upon IL1-beta stimulation (+), before (T0) and after 1 month of ruxolitinib (T1). ( E ) Fold-decrease [(O.D.CCL2/O.D.GAPDH)IL 1beta/[(O.D.CCL2/O.D.GAPDH)UNTR, relative western blot optical density (O.D.) values] of CCL2 protein expression upon ex vivo IL1-beta stimulation of MNCs from PMF 3 patients before (T0) and after 1 month (T1) of ruxolitinib therapy. Data
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 11 Post-mortem evaluation of GRPs' graft localization in C57BL/6 allogenic model without immunosuppression applied: Immunohistochemical staining in the brain of C57BL/6 (allogeneic) mice after 14 days of observation in a group with no immunosuppression applied. The staining revealed the presence of MCP-1 in the tissue ( A ). Singular GFP+ cells aggregates were observed in tissue parenchyma surrounded by TMEM119+ microglia ( B ). The bottom picture shows infiltration of CD45+ cells into artery epithelium ( E ). Singular CD3+ cells were also present in brain parenchyma, mostly in the cerebellum, brainstem, and medulla oblongata areas ( C , D ). Abbreviations: GFP-green fluorescent protein; GRP-glial-restricted progenitor cell.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 rHsGal-1 treatment upregulates anti-inflammatory cytokines. ( A ) Cytokine array expression from NT A/J -/- myotubes cultured in differentiation media for 48 h. ( B ) Cytokine array expression from A/J -/- myotubes after 48 h in differentiation media supplemented with 0.11 muM rHsGal-1. ( C ) Mean pixel density of relative cytokine expression from A and B. ( D ) Schematic reference of the significantly upregulated cytokines after 48 h treatment with 0.11 muM rHsGal-1. ( E ) Quantification of Western blot probing for IL-4. ( F ) Quantification of Western blot of MCP-1 levels normalized to beta-actin in cells treated with PBS (control) or 2.7 mg/kg rHsGal-1. ( G ) Quantification of Western blot of TIMP-2 normalized to GAPDH levels in cells treated with PBS (control) or 2.7 mg/kg rHsGal-1. Tissues taken from mice treated for 1 month. ( H ) Images of Western blot for TIMP-2, MCP-1, beta-actin, and GAPDH on tissues from Bla/J mice treated with PBS (control) or 2.7 mg/kg rHsGal-1 for one month. n = 4 for each group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 NT vs. rHsGal-1. Bars are represented as SEM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 2 Assessment of renal inflammation in human kidney biopsies. A Immunostaining using specific antibodies for different immune cells, including T lymphocytes (CD3 + cells), B lymphocytes (CD20 + cells) and monocytes/macrophages (CD68 + cells), was performed on normal subjects, MCD patients, and DN patients. Original magnification, x400; scale bar, 50 mum. Immunohistochemical staining of the proinflammatory marker, chemokine (C-C motif) ligand 2 (CCL-2) was also performed. Original magnification, x200; scale bar, 50 mum. B - D The numbers of CD3 + , CD20 + , and CD68 + cells per high-power field (HPF) were counted in the tubulointerstitium. *** P < 0.001, * P < 0.05. E Quantitative analysis of the expression of CCL-2 staining on kidney sections of each group according to the average IOD. ** P < 0.01. Immunostaining was performed in duplicate. Significant differences were determined by unpaired two-tailed t -tests. F Correlation of Wnt5a and CD146 expression with inflammatory process and known prognostic factors (percentage of IFTA, renal function, and proteinuria) in patients with diabetic nephropathy. Data are r value based on Pearson and Spearman correlation analysis; ( P value); NS is not significant. IFTA, interstitial fibrosis and tubular atrophy; Estimated GFR, Estimated glomerular filtration rate; HbA1c, hemoglobin A1c.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 8 Immunohistochemical analysis of RPW processed with different antibodies: ( A ) anti-CD36, ( B ) anti-CCL2 and ( C ) anti-TNF-alpha. CHOW: Standard diet; DIO: High-Fat diet; DS: High-Fat diet + Seeds; DJS: High-Fat diet + Juice and Seeds. The densitometric analysis is expressed as an arbitrary optical density unit (ODU). Data are the mean +- SEM. * p < 0.05 vs. CHOW rats; # p < 0.05 vs. DIO rats.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 8 TGF-beta1-increased MCP-1 expression in myofibroblasts is inhibited by both IL-15 treatments. Intracellular expression of MCP-1 was analyzed by flow cytometry in 48 h-treated myofibroblasts with TGF-beta1 (2.5 ng/mL). Left, Representative staining of one experiment is shown. The dark-colored histograms correspond to cells incubated with an anti-MCP-1 antibody, and gray histograms correspond to cells incubated with the isotype-matched control antibody. Mean fluorescence intensity values are shown at the right of each histogram. Right, Graphs represent the relative expression of MCP-1 in four independent experiments. Data are shown as means +- SEM (* p < 0.05, *** p < 0.001, n = 4).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
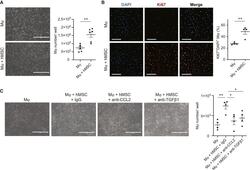
- Experimental details
- Enhanced Mph proliferation by hAM-MSCs via CCL2 and TGF-beta1 pathways Mouse bone marrow-derived Mph were cultured with or without hAM-MSCs in a non-contact co-culture model for 48 h. (A) Phase-contrast images showing the Mph frequency. Mph were dissociated for cell number counts, the results of which are presented in the chart. Scale bars, 400 mum. n = 6. (B) Immunocytolabeling showing Ki67 expression in Mph. Collected Mph were stained for a proliferation marker Ki67 and DAPI, and percentage of Ki67 + nuclei was measured and present in the chart. Scale bars, 50 mum. n = 4 in each group. (C) Effects of inhibition of human CCL2 and TGF-beta1 on hAM-MSC-mediated Mph proliferation. Increased Mph numbers by hAM-MSC co-culture were eliminated by addition of neutralizing antibodies for hCCL2 (Mph + hMSC + anti-CCL2 group) and TGF-beta1 (Mph + hMSC + anti-TGF-beta1 group). Isotype (IgG) antibody used as control. Representative phase-contrast images and a chart summarizing the data are presented. Scale bars, 400 mum. n = 4. Data are presented as mean +- SEM. *p < 0.05 and **p < 0.01. Student's t test (A and B) or one-way ANOVA with Tukey's post hoc test (C).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Enhanced migration of monocytes and Mph by hAM-MSCs through CCL2 (A-D) Migration ability was assessed in freshly isolated mouse bone marrow-derived monocytes (A and C) or Mph (B and D) by seeding in the upper well of a Transwell insert and measuring movement of these cells toward a confluent monolayer of hAM-MSCs (hMSC group) or no cell (media group) in the lower side of the membrane. Effects of co-culture with hAM-MSCs with human CCL2 neutralizing antibody (hMSC + anti-CCL2 group) and administration of recombinant CCL2 with or without anti-CCL2 antibody (CCL2 + anti-CCL2 and CCL2 groups) were also assessed. Migrated cells through the insert membrane were fixed and stained with DAPI and counted. Representative DAPI-staining images (A and B) and respective counts of migrated cell numbers are presented in the charts (C and D). Scale bars, 50 mum. n = 4-5 independent experiments. Conditions were repeated in duplicate for each biological replicate, and an average >5 frames per insert were captured. Data are presented as mean +- SEM. *p < 0.05 and **p < 0.01. One-way ANOVA with Tukey's post hoc test for multiple paired comparisons.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Sodium selenite induces epithin/PRSS14 shedding and CCL2 secretion. (A) 4T1 cells were treated with increasing concentrations of selenite for 4 h and epithin/PRSS14 shedding was analyzed. PMA treatment was used as a positive control. IB, immunoblot. (B) The degrees of epithin/PRSS14 shedding in cells treated with 0 and 2 mug/ml selenite were normalized against that in PMA-treated cells and are shown in scatter plots. Data are presented as mean +- SE. ** P < 0.01 (Student's t -test, n = 5). (C) 4T1 cells grown under DMEM or IMDM condition were treated with sodium selenite for overnight. The degree of CCL2 release was measured by western blot. (D and E) The degree of CCL2 secretion induced by selenite compared to lipopolysaccharide (LPS) was analyzed and quantified as in (B). *** P < 0.001 (Tukey's multiple comparison test). (F) The degrees of selenite-induced CCL2 secretion in 4T1 and 4T1-EpiKD cells were analyzed. (G) Quantification analysis of CCL2 secretion in (F) normalized cells against that from selenite-treated 4T1 cells is shown. *** P < 0.001 (Tukey's multiple comparison test, n = 3). (H) 4T1 cells treated with selenite and/or N-acetylcysteine (NAC) for indicated times were analyzed for their epithin/PRSS14 shedding and CCL2 secretion.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Exosomes containing EBV products promote fibroblast activation and NPC cell growth. A Phenotypic changes in fibroblasts. Primary fibroblasts were treated with PBS or exosomes (10 mug/ml) derived from HK1 cells or HK1EBV cells. Images were captured 24 hours post-exosome stimulation. Scale bar: 50 mum. B Real-time monitoring of changes in the fibroblasts stimulated with exosomes derived from HK1 cells, HK1EBV cells, or PBS (control). The black arrow indicates the time of exosome addition. Data are presented as the mean and SD of triplicate experiments. C Assessment of exosome-mediated effects on fibroblast contractility. Fibroblasts were treated with exosomes derived from HK1 or HK1EBV cells or medium only (control) for 3 days (left). Quantification of contractility was determined by measuring the gel area using ImageJ. Data are presented as the mean and SD (*p < 0.05, **p < 0.01, ***p < 0.001; paired t test). Scale bar, 2 mm. D Real-time proliferation assay of the NPC cells treated with cancer cell culture medium, culture supernatants harvested from the fibroblasts treated with HK1EBV cell-derived exosomes (Exo-Tx Fibro CM ), or untreated fibroblasts (Fibro CM ). Data are presented as the mean and SD of triplicate experiments. E Analysis of EdU incorporation in the NPC-TW06 cells treated with supernatants harvested from the fibroblasts treated with HK1EBV cell-derived exosomes or control medium for 10 hours, followed by EdU labeling (green) for 4 hours. Scale bar, 100 mum. EdU
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA