32-4800
antibody from Invitrogen Antibodies
Targeting: PTBP1
HNRNP-I, HNRPI, pPTB, PTB, PTB-1, PTB2, PTB3, PTB4
Antibody data
- Antibody Data
- Antigen structure
- References [42]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [2]
- Flow cytometry [1]
- Other assay [53]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 32-4800 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- PTBP1 Monoclonal Antibody (1)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- This antibody reacts with the C-terminus of the human and rodent PTB protein.
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 1
- Vial size
- 100 µg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references The selenoprotein P 3' untranslated region is an RNA binding protein platform that fine tunes selenocysteine incorporation.
Histone marks regulate the epithelial-to-mesenchymal transition via alternative splicing.
Essential requirement for polypyrimidine tract binding proteins 1 and 3 in the maturation and maintenance of mature B cells in mice.
The human nucleoporin Tpr protects cells from RNA-mediated replication stress.
Identification of human long noncoding RNAs associated with nonalcoholic fatty liver disease and metabolic homeostasis.
The mTOR regulated RNA-binding protein LARP1 requires PABPC1 for guided mRNA interaction.
Tumor suppressor SMAR1 regulates PKM alternative splicing by HDAC6-mediated deacetylation of PTBP1.
RNA-binding protein Ptbp1 regulates alternative splicing and transcriptome in spermatogonia and maintains spermatogenesis in concert with Nanos3.
SINEUP long non-coding RNA acts via PTBP1 and HNRNPK to promote translational initiation assemblies.
Synthetic in vitro transcribed lncRNAs (SINEUPs) with chemical modifications enhance target mRNA translation.
Intronic Determinants Coordinate Charme lncRNA Nuclear Activity through the Interaction with MATR3 and PTBP1.
Internal Ribosome Entry Site Dramatically Reduces Transgene Expression in Hematopoietic Cells in a Position-Dependent Manner.
The Long Noncoding RNA Pnky Is a Trans-acting Regulator of Cortical Development In Vivo.
H19 potentiates let-7 family expression through reducing PTBP1 binding to their precursors in cholestasis.
The Long Noncoding RNA Lncenc1 Maintains Naive States of Mouse ESCs by Promoting the Glycolysis Pathway.
A Short Tandem Repeat-Enriched RNA Assembles a Nuclear Compartment to Control Alternative Splicing and Promote Cell Survival.
The RNA-binding protein PTBP1 is necessary for B cell selection in germinal centers.
PolyC-binding proteins enhance expression of the CDK2 cell cycle regulatory protein via alternative splicing.
hnRNP I regulates neonatal immune adaptation and prevents colitis and colorectal cancer.
Pathogenic variants that alter protein code often disrupt splicing.
Long noncoding RNA MEG3 induces cholestatic liver injury by interaction with PTBP1 to facilitate shp mRNA decay.
A mechanism underlying position-specific regulation of alternative splicing.
The alternative splicing program of differentiated smooth muscle cells involves concerted non-productive splicing of post-transcriptional regulators.
Characterization of the Regulation of CD46 RNA Alternative Splicing.
Human polypyrimidine tract-binding protein interacts with mitochondrial tRNA(Thr) in the cytosol.
Methods for the characterization of stress granules in virus infected cells.
Brain metastasis is predetermined in early stages of cutaneous melanoma by CD44v6 expression through epigenetic regulation of the spliceosome.
ABLIM1 splicing is abnormal in skeletal muscle of patients with DM1 and regulated by MBNL, CELF and PTBP1.
PTB deficiency causes the loss of adherens junctions in the dorsal telencephalon and leads to lethal hydrocephalus.
Psip1/Ledgf p52 binds methylated histone H3K36 and splicing factors and contributes to the regulation of alternative splicing.
Translation of Myocyte Enhancer Factor-2 is induced by hypertrophic stimuli in cardiomyocytes through a Calcineurin-dependent pathway.
The leader protein of cardioviruses inhibits stress granule assembly.
MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy.
Polypyrimidine tract-binding protein regulates the cell cycle through IRES-dependent translation of CDK11(p58) in mouse embryonic stem cells.
In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs.
Polypyrimidine tract-binding protein 1 regulates the alternative splicing of dopamine receptor D2.
PTBP1 is required for embryonic development before gastrulation.
Regulation of alternative splicing by histone modifications.
Cardiovirus leader proteins are functionally interchangeable and have evolved to adapt to virus replication fitness.
Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis.
Alteration of cellular RNA splicing and polyadenylation machineries during productive human cytomegalovirus infection.
The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins.
Shetty SP, Kiledjian NT, Copeland PR
PloS one 2022;17(7):e0271453
PloS one 2022;17(7):e0271453
Histone marks regulate the epithelial-to-mesenchymal transition via alternative splicing.
Segelle A, Núñez-Álvarez Y, Oldfield AJ, Webb KM, Voigt P, Luco RF
Cell reports 2022 Feb 15;38(7):110357
Cell reports 2022 Feb 15;38(7):110357
Essential requirement for polypyrimidine tract binding proteins 1 and 3 in the maturation and maintenance of mature B cells in mice.
Monzón-Casanova E, Bates KJ, Smith CWJ, Turner M
European journal of immunology 2021 Sep;51(9):2266-2273
European journal of immunology 2021 Sep;51(9):2266-2273
The human nucleoporin Tpr protects cells from RNA-mediated replication stress.
Kosar M, Giannattasio M, Piccini D, Maya-Mendoza A, García-Benítez F, Bartkova J, Barroso SI, Gaillard H, Martini E, Restuccia U, Ramirez-Otero MA, Garre M, Verga E, Andújar-Sánchez M, Maynard S, Hodny Z, Costanzo V, Kumar A, Bachi A, Aguilera A, Bartek J, Foiani M
Nature communications 2021 Jun 24;12(1):3937
Nature communications 2021 Jun 24;12(1):3937
Identification of human long noncoding RNAs associated with nonalcoholic fatty liver disease and metabolic homeostasis.
Ruan X, Li P, Ma Y, Jiang CF, Chen Y, Shi Y, Gupta N, Seifuddin F, Pirooznia M, Ohnishi Y, Yoneda N, Nishiwaki M, Dumbovic G, Rinn JL, Higuchi Y, Kawai K, Suemizu H, Cao H
The Journal of clinical investigation 2021 Jan 4;131(1)
The Journal of clinical investigation 2021 Jan 4;131(1)
The mTOR regulated RNA-binding protein LARP1 requires PABPC1 for guided mRNA interaction.
Smith EM, Benbahouche NEH, Morris K, Wilczynska A, Gillen S, Schmidt T, Meijer HA, Jukes-Jones R, Cain K, Jones C, Stoneley M, Waldron JA, Bell C, Fonseca BD, Blagden S, Willis AE, Bushell M
Nucleic acids research 2021 Jan 11;49(1):458-478
Nucleic acids research 2021 Jan 11;49(1):458-478
Tumor suppressor SMAR1 regulates PKM alternative splicing by HDAC6-mediated deacetylation of PTBP1.
Choksi A, Parulekar A, Pant R, Shah VK, Nimma R, Firmal P, Singh S, Kundu GC, Shukla S, Chattopadhyay S
Cancer & metabolism 2021 Apr 16;9(1):16
Cancer & metabolism 2021 Apr 16;9(1):16
RNA-binding protein Ptbp1 regulates alternative splicing and transcriptome in spermatogonia and maintains spermatogenesis in concert with Nanos3.
Senoo M, Hozoji H, Ishikawa-Yamauchi Y, Takijiri T, Ohta S, Ukai T, Kabata M, Yamamoto T, Yamada Y, Ikawa M, Ozawa M
The Journal of reproduction and development 2020 Oct 13;66(5):459-467
The Journal of reproduction and development 2020 Oct 13;66(5):459-467
SINEUP long non-coding RNA acts via PTBP1 and HNRNPK to promote translational initiation assemblies.
Toki N, Takahashi H, Sharma H, Valentine MNZ, Rahman FM, Zucchelli S, Gustincich S, Carninci P
Nucleic acids research 2020 Nov 18;48(20):11626-11644
Nucleic acids research 2020 Nov 18;48(20):11626-11644
Synthetic in vitro transcribed lncRNAs (SINEUPs) with chemical modifications enhance target mRNA translation.
Toki N, Takahashi H, Zucchelli S, Gustincich S, Carninci P
FEBS letters 2020 Dec;594(24):4357-4369
FEBS letters 2020 Dec;594(24):4357-4369
Intronic Determinants Coordinate Charme lncRNA Nuclear Activity through the Interaction with MATR3 and PTBP1.
Desideri F, Cipriano A, Petrezselyova S, Buonaiuto G, Santini T, Kasparek P, Prochazka J, Janson G, Paiardini A, Calicchio A, Colantoni A, Sedlacek R, Bozzoni I, Ballarino M
Cell reports 2020 Dec 22;33(12):108548
Cell reports 2020 Dec 22;33(12):108548
Internal Ribosome Entry Site Dramatically Reduces Transgene Expression in Hematopoietic Cells in a Position-Dependent Manner.
Zheng Q, Zhang X, Yang H, Xie J, Xie Y, Chen J, Yu C, Zhong C
Viruses 2019 Oct 8;11(10)
Viruses 2019 Oct 8;11(10)
The Long Noncoding RNA Pnky Is a Trans-acting Regulator of Cortical Development In Vivo.
Andersen RE, Hong SJ, Lim JJ, Cui M, Harpur BA, Hwang E, Delgado RN, Ramos AD, Liu SJ, Blencowe BJ, Lim DA
Developmental cell 2019 May 20;49(4):632-642.e7
Developmental cell 2019 May 20;49(4):632-642.e7
H19 potentiates let-7 family expression through reducing PTBP1 binding to their precursors in cholestasis.
Zhang L, Yang Z, Huang W, Wu J
Cell death & disease 2019 Feb 18;10(3):168
Cell death & disease 2019 Feb 18;10(3):168
The Long Noncoding RNA Lncenc1 Maintains Naive States of Mouse ESCs by Promoting the Glycolysis Pathway.
Sun Z, Zhu M, Lv P, Cheng L, Wang Q, Tian P, Yan Z, Wen B
Stem cell reports 2018 Sep 11;11(3):741-755
Stem cell reports 2018 Sep 11;11(3):741-755
A Short Tandem Repeat-Enriched RNA Assembles a Nuclear Compartment to Control Alternative Splicing and Promote Cell Survival.
Yap K, Mukhina S, Zhang G, Tan JSC, Ong HS, Makeyev EV
Molecular cell 2018 Nov 1;72(3):525-540.e13
Molecular cell 2018 Nov 1;72(3):525-540.e13
The RNA-binding protein PTBP1 is necessary for B cell selection in germinal centers.
Monzón-Casanova E, Screen M, Díaz-Muñoz MD, Coulson RMR, Bell SE, Lamers G, Solimena M, Smith CWJ, Turner M
Nature immunology 2018 Mar;19(3):267-278
Nature immunology 2018 Mar;19(3):267-278
PolyC-binding proteins enhance expression of the CDK2 cell cycle regulatory protein via alternative splicing.
Ji X, Humenik J, Yang D, Liebhaber SA
Nucleic acids research 2018 Feb 28;46(4):2030-2044
Nucleic acids research 2018 Feb 28;46(4):2030-2044
hnRNP I regulates neonatal immune adaptation and prevents colitis and colorectal cancer.
Jin Z, Liang F, Yang J, Mei W
PLoS genetics 2017 Mar;13(3):e1006672
PLoS genetics 2017 Mar;13(3):e1006672
Pathogenic variants that alter protein code often disrupt splicing.
Soemedi R, Cygan KJ, Rhine CL, Wang J, Bulacan C, Yang J, Bayrak-Toydemir P, McDonald J, Fairbrother WG
Nature genetics 2017 Jun;49(6):848-855
Nature genetics 2017 Jun;49(6):848-855
Long noncoding RNA MEG3 induces cholestatic liver injury by interaction with PTBP1 to facilitate shp mRNA decay.
Zhang L, Yang Z, Trottier J, Barbier O, Wang L
Hepatology (Baltimore, Md.) 2017 Feb;65(2):604-615
Hepatology (Baltimore, Md.) 2017 Feb;65(2):604-615
A mechanism underlying position-specific regulation of alternative splicing.
Hamid FM, Makeyev EV
Nucleic acids research 2017 Dec 1;45(21):12455-12468
Nucleic acids research 2017 Dec 1;45(21):12455-12468
The alternative splicing program of differentiated smooth muscle cells involves concerted non-productive splicing of post-transcriptional regulators.
Llorian M, Gooding C, Bellora N, Hallegger M, Buckroyd A, Wang X, Rajgor D, Kayikci M, Feltham J, Ule J, Eyras E, Smith CW
Nucleic acids research 2016 Oct 14;44(18):8933-8950
Nucleic acids research 2016 Oct 14;44(18):8933-8950
Characterization of the Regulation of CD46 RNA Alternative Splicing.
Tang SJ, Luo S, Ho JXJ, Ly PT, Goh E, Roca X
The Journal of biological chemistry 2016 Jul 1;291(27):14311-14323
The Journal of biological chemistry 2016 Jul 1;291(27):14311-14323
Human polypyrimidine tract-binding protein interacts with mitochondrial tRNA(Thr) in the cytosol.
Marnef A, Jády BE, Kiss T
Nucleic acids research 2016 Feb 18;44(3):1342-53
Nucleic acids research 2016 Feb 18;44(3):1342-53
Methods for the characterization of stress granules in virus infected cells.
Panas MD, Kedersha N, McInerney GM
Methods (San Diego, Calif.) 2015 Nov 15;90:57-64
Methods (San Diego, Calif.) 2015 Nov 15;90:57-64
Brain metastasis is predetermined in early stages of cutaneous melanoma by CD44v6 expression through epigenetic regulation of the spliceosome.
Marzese DM, Liu M, Huynh JL, Hirose H, Donovan NC, Huynh KT, Kiyohara E, Chong K, Cheng D, Tanaka R, Wang J, Morton DL, Barkhoudarian G, Kelly DF, Hoon DS
Pigment cell & melanoma research 2015 Jan;28(1):82-93
Pigment cell & melanoma research 2015 Jan;28(1):82-93
ABLIM1 splicing is abnormal in skeletal muscle of patients with DM1 and regulated by MBNL, CELF and PTBP1.
Ohsawa N, Koebis M, Mitsuhashi H, Nishino I, Ishiura S
Genes to cells : devoted to molecular & cellular mechanisms 2015 Feb;20(2):121-34
Genes to cells : devoted to molecular & cellular mechanisms 2015 Feb;20(2):121-34
PTB deficiency causes the loss of adherens junctions in the dorsal telencephalon and leads to lethal hydrocephalus.
Shibasaki T, Tokunaga A, Sakamoto R, Sagara H, Noguchi S, Sasaoka T, Yoshida N
Cerebral cortex (New York, N.Y. : 1991) 2013 Aug;23(8):1824-35
Cerebral cortex (New York, N.Y. : 1991) 2013 Aug;23(8):1824-35
Psip1/Ledgf p52 binds methylated histone H3K36 and splicing factors and contributes to the regulation of alternative splicing.
Pradeepa MM, Sutherland HG, Ule J, Grimes GR, Bickmore WA
PLoS genetics 2012;8(5):e1002717
PLoS genetics 2012;8(5):e1002717
Translation of Myocyte Enhancer Factor-2 is induced by hypertrophic stimuli in cardiomyocytes through a Calcineurin-dependent pathway.
Ye J, Cardona M, Llovera M, Comella JX, Sanchis D
Journal of molecular and cellular cardiology 2012 Oct;53(4):578-87
Journal of molecular and cellular cardiology 2012 Oct;53(4):578-87
The leader protein of cardioviruses inhibits stress granule assembly.
Borghese F, Michiels T
Journal of virology 2011 Sep;85(18):9614-22
Journal of virology 2011 Sep;85(18):9614-22
MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy.
Smith PY, Delay C, Girard J, Papon MA, Planel E, Sergeant N, Buée L, Hébert SS
Human molecular genetics 2011 Oct 15;20(20):4016-24
Human molecular genetics 2011 Oct 15;20(20):4016-24
Polypyrimidine tract-binding protein regulates the cell cycle through IRES-dependent translation of CDK11(p58) in mouse embryonic stem cells.
Ohno S, Shibayama M, Sato M, Tokunaga A, Yoshida N
Cell cycle (Georgetown, Tex.) 2011 Nov 1;10(21):3706-13
Cell cycle (Georgetown, Tex.) 2011 Nov 1;10(21):3706-13
In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs.
Smith P, Al Hashimi A, Girard J, Delay C, Hébert SS
Journal of neurochemistry 2011 Jan;116(2):240-7
Journal of neurochemistry 2011 Jan;116(2):240-7
Polypyrimidine tract-binding protein 1 regulates the alternative splicing of dopamine receptor D2.
Sasabe T, Futai E, Ishiura S
Journal of neurochemistry 2011 Jan;116(1):76-81
Journal of neurochemistry 2011 Jan;116(1):76-81
PTBP1 is required for embryonic development before gastrulation.
Suckale J, Wendling O, Masjkur J, Jäger M, Münster C, Anastassiadis K, Stewart AF, Solimena M
PloS one 2011 Feb 17;6(2):e16992
PloS one 2011 Feb 17;6(2):e16992
Regulation of alternative splicing by histone modifications.
Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T
Science (New York, N.Y.) 2010 Feb 19;327(5968):996-1000
Science (New York, N.Y.) 2010 Feb 19;327(5968):996-1000
Cardiovirus leader proteins are functionally interchangeable and have evolved to adapt to virus replication fitness.
Paul S, Michiels T
The Journal of general virology 2006 May;87(Pt 5):1237-1246
The Journal of general virology 2006 May;87(Pt 5):1237-1246
Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis.
Knoch KP, Bergert H, Borgonovo B, Saeger HD, Altkrüger A, Verkade P, Solimena M
Nature cell biology 2004 Mar;6(3):207-14
Nature cell biology 2004 Mar;6(3):207-14
Alteration of cellular RNA splicing and polyadenylation machineries during productive human cytomegalovirus infection.
Adair R, Liebisch GW, Su Y, Colberg-Poley AM
The Journal of general virology 2004 Dec;85(Pt 12):3541-3553
The Journal of general virology 2004 Dec;85(Pt 12):3541-3553
The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins.
Delhaye S, van Pesch V, Michiels T
Journal of virology 2004 Apr;78(8):4357-62
Journal of virology 2004 Apr;78(8):4357-62
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
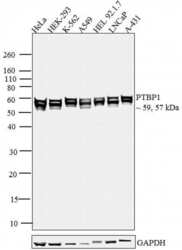
- Experimental details
- Western blot analysis was performed on nuclear enriched cell extracts of HeLa (Lane 1), HEK-293 (Lane 2), K-562 (Lane 3), A549 (Lane 4), HEL 92.1.7 (Lane 5), LNCaP (Lane 6) and A-431 (Lane 7). The blots were probed with Anti- PTBP1 Mouse Monoclonal Antibody (Product # 32-4800, 2 µg/mL) and detected by chemiluminescence using Goat anti-Mouse IgG (H+L) Superclonal Secondary Antibody, HRP conjugate (Product # A28177, 0.4 µg/mL, 1:2500 dilution). ~ 59 kDa and ~57 kDa bands corresponding to PTBP1 was observed across all cell lines tested. Known quantity of protein samples were electrophoresed using Novex® NuPAGE® 4-12 % Bis-Tris gel (Product # NP0321BOX), XCell SureLock Electrophoresis System (Product # EI0002) and Novex® Sharp Pre-Stained Protein Standard (Product # LC5800). Resolved proteins were then transferred onto a nitrocellulose membrane with iBlot® 2 Dry Blotting System (Product # IB21001). The membrane was probed with the relevant primary and secondary Antibody following blocking with 5 % skimmed milk. Chemiluminescent detection was performed using Pierce™ ECL Western Blotting Substrate (Product # 32106).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Knockdown of PTBP1 was achieved by transfecting HeLa cells with PTBP1 specific validated siRNAs (Silencer® select Product # s11434). Western blot analysis (Fig. a) was performed using membrane enriched extracts from the PTBP1 knockdown cells (lane 3), non-specific scrambled siRNA transfected cells (lane 2) and untransfected cells (lane 1). The blots were probed with PTBP1 Monoclonal Antibody (Product # 32-4800, 2 µg/mL) and Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, HRP conjugate (Product # A28177, 0.25µg/mL, 1:4000 dilution). Densitometric analysis of this western blot is shown in histogram (Fig. b). Decrease in signal upon siRNA mediated knock down confirms that antibody is specific to PTBP1 .
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescence analysis of PTB (CLONE 1) was performed using 70% confluent log phase HCT-116 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with PTBP1 / PTB Mouse Monoclonal Antibody (Product # 32-4800) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescence analysis of PTB (CLONE 1) was performed using 70% confluent log phase HCT 116 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with PTBP1/PTB (1) Mouse Monoclonal Antibody (Product # 32-4800) at 2µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Flow cytometry analysis of PTBP1 / PTB was done on HCT 116 cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with PTBP1 / PTB Mouse Monoclonal Antibody (Product # 32-4800, red histogram) or with mouse isotype control (pink histogram) at 3-5 µg/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (Product # A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control..
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunoprecipitation of PTBP1 was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of PTPB1 monoclonal antibody (Product # 324800) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D) and washed extensively. They were then eluted and analyzed using the Jess Simple Western system. Lane 1 is input, lane 2 IP without antibody and lane 3 IP with antibody. Target was detected a PTPB1 monoclonal antibody (Product # 324800) at a dilution of 1:25, followed by a 1:100 dilution of secondary antibody. Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- RNA immunoprecipitation (RIP) western of PTBP1 was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of PTPB1 monoclonal antibody (Product # 324800) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D) and washed extensively. They were then eluted and analyzed using the Jess Simple Western system. Lane 1 is input, lane 2 IP without antibody and lane 3 IP with antibody. Target was detected a PTPB1 monoclonal antibody (Product # 324800) at a dilution of 1:25, followed by a 1:100 dilution of secondary antibody. Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- RNA immunoprecipitation (RIP) western of PTBP1 was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of PTPB1 monoclonal antibody (Product # 324800) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D) and washed extensively. They were then eluted and analyzed using the Jess Simple Western system. Lane 1 is input, lane 2 IP without antibody and lane 3 IP with antibody. Target was detected a PTPB1 monoclonal antibody (Product # 324800) at a dilution of 1:25, followed by a 1:100 dilution of secondary antibody. Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 7 PTBP1 expression in normal and mutant embryos. Comparison of immunofluorescence from PTBP1 and its paralogue PTBP2 in control and PTBP1 KO embryos at E7.5. The top row shows serial cryosections of a normally sized control embryo stained for PTBP1 (left) and PTBP2 (right). PTBP1 was expressed in most embryonic cells, notably the visceral endoderm and the epiblast, comparable to the LacZ reporter staining in Figure 2 . A strong PTBP1 signal was also observed in decidual nuclei. PTBP2 in controls was mostly expressed in the epiblast and absent from most decidual nuclei. PTBP1 embryos (bottom) showed no PTBP1 or PTBP2 signal, displayed here merged with the DAPI signal to reveal the embryo (dashed line). Again most cells surrounding the embryo displayed a nuclear PTBP1 signal while only very few showed a weak nuclear PTBP2 signal (arrow).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1. Human polypyrimidine tract-binding proteins PTB, nPTB and ROD1 interact with mt tRNA Thr . ( A ) Detection of a novel PTB-associated RNA. RNAs co-immunoprecipitated with PTB from a HeLa extract (Ext) were 3' end-labeled and analyzed on a 6% sequencing gel (lower panel). The 5S and 5.8S rRNAs, the U1 snRNA and mt tRNA Thr are indicated. IP of PTB was confirmed by western blot analysis (upper panel). Mock IP performed without antibody (lanes no AB) and molecular size markers (M) are also shown. ( B ) RNAs co-precipitated with transiently expressed Flag-tagged PTB, nPTB and ROD1 proteins. IP was monitored by western blot analysis with anti-Flag antibody (alpha-Flag). NT, non-transfected cells. Other details are identical to panel A. ( C ) RNA chemical sequencing. HeLa mt tRNA Thr co-immunoprecipitated with PTB was 3' end-labeled, purified on a denaturing gel and subjected to chemical sequencing. RNA fragments were fractionated on an 8% sequencing gel. The nucleotide sequence of mt tRNA Thr is indicated and numbered according to ( 54 ). A9 may correspond to 1-methyl-adenosyl ( 40 ), while U61 may be a pseudouridine, since it shows no hydrazine reactivity. The nature of the highly reactive C32 residue is unknown. Lane OH-, hydrolysis ladder. ( D ) Determination of the 3'-terminal sequence of PTB-associated mt tRNA Thr by 3' end race. Sequences of the RNA tag and the 3' end region of mt tRNA Thr are indicated.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3. PTB forms a direct interaction with mt tRNA Thr in HeLa cells. ( A ) In vivo RNA-protein cross-linking. Accumulation of endogenous PTB and transiently expressed FL-PTB in transfected (FL-PTB) and non-transfected (NT) HeLa cells was monitored by western blotting with an anti-PTB antibody (upper panel). Tubulin, loading control. Cells were in vivo cross-linked (x-link) with formaldehyde as indicted above the lanes. After extract (Ext) preparation, FL-PTB was immunoprecipitated under stringent conditions. IP of FL-PTB was confirmed by western blotting with anti-Flag antibody and co-precipitation of mt tRNA Thr and U1 snRNA were tested by northern. ( B ) PTB association with in vitro synthesized mt tRNA Thr introduced into HeLa cells. PTB was immunoprecipitated from extracts (Ext) prepared from HeLa cells non-transfected (NT) or transfected with the indicated amounts of in vitro transcribed mt tRNA Thr and mt tRNA Asp (lower panel). Co-IP of tRNAs was monitored by northern blotting (upper panel). ( C ) Label transfer from mt tRNA Thr to PTB. In vitro transcribed internally labeled mt tRNA Thr and cyt tRNA Thr were incubated with HeLa cell extracts. After UV irradiation, RNAs were hydrolyzed by RNase treatment and PTB was immunoprecipitated and analyzed on an SDS gel. PTB IP was confirmed by western blotting (upper panel) and label transfer was visualized by autoradiography (lower panel). ( D ) Gel shift analysis. In vitro transcribed internally labeled mt tRNA Thr , c-s
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4. RNA and protein elements directing interaction of PTB and mt tRNA Thr . ( A ) The D-stem-loop and T-loop nucleotides of mt tRNA Thr are essential for PTB binding. Internally labeled WT and mutant mt tRNA Thr transcripts were transfected into HeLa cells. After 24 h of incubation, cell extracts (Ext) were prepared, PTB was immunoprecipitated and analyzed by western blotting (lower panel). Co-IP of mt tRNA Thr transcripts was analyzed by fractionation on a 6% sequencing gel. The structure of wild-type mt tRNA Thr and the nucleotide alterations (lower case letters) in the acceptor-stem ( ACS ), antisense-stem ( ASS ), D-stem (DS), T-stem (TS), D-loop (DL), antisense-loop (ASL) and T-loop (TL) of mutant mt tRNA Thr transcripts are shown. Lanes no AB, control IPs without antibody. ( B ) In vivo interaction of mutant PTB proteins with mt tRNA Thr . Schematic structure of human PTB and positions of the RNA recognition motifs (RRM1 to RRM4) are indicated. Flag-tagged terminally truncated PTB proteins (FL-PTBd1 to FL-PTBd5) were transiently expressed in HeLa cells and immunoprecipitated with an anti-Flag antibody. The amino acid alterations carried by the m, b and m + b mutants of FL-PTBd3 are indicated. The IP reactions were analyzed by western (lower panel) and northern (upper panel) blotting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5. Subcellular localization of the PTB/mt tRNA Thr complex. ( A ) PTB and mt tRNA Thr interact in the cytoplasm. PTB was immunoprecipitated from extracts prepared from HeLa cells (Tot) or from the nuclear (Nuc) or cytoplasmic (Cyt) fractions of HeLa cells. IP of PTB and co-precipitation of mt tRNA Thr and 12S rRNA were measured by western and northern blot analyses. Distribution of cytoplasmic (12S rRNA, mt tRNA Lys , ERp72, tubulin, TOM20 and HSP60) and nuclear (U2 snRNA and hnRNP A1) markers is shown. ( B ) PTB interacts with mt tRNA Thr outside of mitochondria. The cytoplasmic fraction of HeLa cells was further fractionated into crude mitochondrial (Mit) and cytosolic (Cytsl) fractions. One fiftieth of the extracts were used to determine the distribution of PTB, mt tRNA Thr and several mitochondrial (12S rRNA, Cyt c, ATP5A1 and TOM20) and cytosolic (7SL RNA, tubulin, ERp72) markers (lanes 1-3). From the remaining cytoplasmic, cytosolic and mitochondrial extracts, PTB was immunoprecipitated and co-precipitation of mt tRNA Thr , 12S rRNA and 7SL RNA was measured (lanes 4, 7 and 9). Lanes no AB, control IPs without antibody. ( C ) PTB is not required for mt tRNA Thr expression. Accumulation of mt tRNA Thr was measured in HeLa cells treated with control, PTB-specific or PTB- and nPTB-specific interfering RNAs. PTB and nPTB accumulation was measured by western blotting. ERp72 and U1 snRNA were used as loading controls. ( D ) PTB activity is not required for mitochondrial
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 6. Increased PTB association with mt tRNA Thr in apoptotic cells. ( A ) Interaction of PTB and mt tRNA Thr during apoptosis. PTB was immunoprecipitated from extracts (Ext) prepared from HeLa cells treated with staurosporine as indicated. Accumulation of PTB and PARP-1 (lanes 1-4) and IP of PTB (lanes 6-9) was monitored by western blotting. Caspase-cleaved PTB (c-PTB) and PARP1 (c-PARP) are indicated. Co-IP of mt tRNA Thr and U1 snRNA was measured by northern blotting. ( B ) Caspase inhibition in apoptotic cells has no effect on PTB and mt tRNA Thr association. PTB was immunoprecipitated from extracts prepared from HeLa cells treated with z-DEVD-FMK and staurosporine as indicated. Other details are identical to panel A.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig 1 Generation of the IEC-specific hnRNPI knockout mice. (A) A schematic representation shows the knockout strategy. A primer pair (F1 and B1) flanking one loxP site (arrowheads) was used for genotyping the IEC-specific hnRNPI knockout mice. (B) An example of genotyping PCR result shows the sizes of PCR products amplified from the hnRNPI floxed allele (236 bp) and wild-type allele (166 bp). *, 50bp DNA ladder. (C) Western blotting result shows efficient ablation of hnRNPI in the colonic epithelial cells of the knockout mice. hnRNPI protein levels from two knockout mice and two control sibling littermates are shown. (D) and (E) Immunofluorescence staining using an anti-hnRNPI antibody shows hnRNPI protein localization in the wild-type and hnRNPI knockout colon. Magnified images are shown in the lower panels. Nuclear accumulation of hnRNPI protein was detected in both colonic epithelial cells (arrowheads) and cells in the lamina propria (arrows) in wild-type mice (D). hnRNPI expression is diminished in the nuclei of colonic epithelial cells in the knockout mice, but remains unaffected in the lamina propria cells (E, arrows). Note the increased number of immune cells in the lamina propria of the knockout mice. The dotted line indicates the border of a centrally located crypt. Nuclei were counterstained with DAPI. WT, wild-type; KO, knockout. Scale bars, 50 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 7. Divergent levels of U1 snRNP proteins and U1 snRNA during phenotypic modulation. ( A ) Western blots for different splicing factors and RNA binding proteins in mouse aorta and bladder samples, comparing differentiated (D) and proliferative (P) samples. Acta2 and Myh-11 antibodies are markers of smooth muscle differentiation. Rpb1, Tubulin and Gapdh are loading controls. snRNA expression levels in mouse ( B ) and rat PAC1 cells ( C ) measured by qPCR. Primers for snRNA levels were normalized against the same set of genes as in Figures 5 and 6 . Error bars represent standard deviation of the mean ( n = 3). Statistically significance was calculated using Student's t -test, and is shown * P < 0.05. ( D ) RT-PCR for Snrp70 (qPCR with primers as in Figure 6 ) and Actn1 and Tpm1 (with primers as in Figure 2 ) in P and D Rat PAC1 cells. Error bars represent mean and standard deviation, ( n = 3). ( E ) Left panels: RNA-FISH for U1 snRNA in rat PAC1 D and P cells. Right panels show DAPI staining for nuclei. ( F ) Immunofluorescence in rat PAC1 cells for SNRNP70 and U1C. Sm Actin is a marker of SMC differentiation.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 PNCTR Is a pol-I Transcript Interacting with Multiple Copies of PTBP1 Protein (A) Diagram of the predicted PNCTR locus also showing an adjacent 47S/45S rRNA gene and probes used in this study. Mapping to chr21 should be considered provisional since different IGS sequences share extensive regions of homology, and not all parts of human rDNA have been sequenced. (B) Top: northern blot analysis of PNCTR expression in HeLa cells using the probe introduced in (A). Bottom: methylene-blue-stained membrane showing that the lanes were loaded equally. (C) RIP carried out with a PTBP1-specific antibody or a non-immune IgG control. Immunoprecipitated RNAs were analyzed by qRT-PCR using primers specific to PNCTR, PTBP2 pre-mRNA (positive control), or U6 snRNA (negative control). Data are averaged from three experiments +- SD and compared by a two-tailed t test. (D) EMSA with purified PTBP1 protein and a PNCTR-specific RNA probe (sequence on the top). Bottom right: multivalent complexes assemble on incubating the probe with increasing amounts of PTBP1. Bottom left: no band shifts are detected when PTBP1 is substituted with BSA. (E) The PTBP1-PNCTR interaction in (D) is specific since it can be disrupted by increasing amounts of unlabeled PNCTR probe (bottom left), but not a control competitor (top, control RNA sequence; bottom right, the EMSA result). (F) IF-FISH staining of HeLa cells showing that PNCTR co-localizes with PTBP1 in the perinucleolar compartment (PNC). FBL, nucl
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 PNCTR Recruits PTBP1 to the PNC (A) HeLa cells were treated for 24 hr with 400 nM gmControl or gmPNCTR and co-stained with a PTBP1-specific antibody and a PNCTR-specific FISH probe. In most nuclei, gmPNCTR eliminates PNC-localized signals in both the PNCTR and PTBP1 channels. (B) Comparison of the dot areas in individual nuclei in (A) using a two-sided Kolmogorov-Smirnov (KS) test. (C) Two-dimensional density plots summarizing the relationship between PNCTR and PTBP1 foci in (A). (D) HeLa cells were incubated with either siControl or siPTBP1 for 48 hr and analyzed by IF-FISH as in (A). Note that siPTBP1 diminishes the size and intensity of both the PTBP1 and the PNCTR signals, but PNCTR is affected to a lesser extent than PTBP1. Scale bars in (A) and (D), 10 mum. (E) Comparison of the dot areas in (D) using a two-sided KS test. (F) Two-dimensional density plots for the relationship between PNCTR and PTBP1 foci in (D). Maximal densities in (C) and (F) were set to 1. See also Figures S3 and S4 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Quantitative Analysis of PTBP1 Sequestration by PNCTR (A) Co-staining untreated HeLa cells with antibodies against PTBP1 and a PNCTR-specific single-molecule FISH (smFISH) probe set confirms that PNCTR and PTBP1 co-localize in the PNC. Scale bar, 10 mum. (B) Inspection of magnified image in (A) additionally reveals individual PNCTR molecules occurring as diffraction-limited spots near the PNC in interphase nuclei (i1 and i2) or distributed throughout DAPI-positive area in cells entering mitosis (m). Arrowheads mark examples of PNCTR molecules co-localizing with PTBP1. Scale bar, 1 mum. (C) Individual PNCTR molecules in (B) give rise to relatively uniform FISH signal intensities. (D) PNCTR abundance calculated as a ratio between the total PNCTR fluorescence per interphase nucleus in (A) and the median intensity of individual PNCTR molecules from (C). (E) Fraction of PTBP1 co-localizing with PNCTR in interphase nuclei in (A). In (C)-(E), solid teal lines show kernel density estimates for the histogram data, and dashed teal lines mark the medians. See also Figure S5 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 6 Lncenc1 Associates with HNRNPK and PTBP1 Functionally (A) Results of RNA pull-down experiments. Biotinylated Lncenc1 sense or antisense RNAs were used for the experiments. Two specific bands (arrowheads) were excised and subjected to mass spectrometry analysis, and were further validated by western blotting. (B) RNA immunoprecipitation (RIP) with anti-HNRNPK and anti-PTBP1 antibodies in E14 nESCs. Enrichments of lncRNAs, i.e., expression of RIP relative to input samples, were determined by qRT-PCR. (C and D) Expression of pluripotency genes (C) or glycolysis genes (D) upon knockdown of Ptbp1 and Hnrnpk . Data were normalized against Actb , and shown as the means +- SD of three independent experiments. Statistical significance of t test: * p < 0.05, ** p < 0.01, *** p < 0.001. (E) Glycolysis signatures of Hnrnpk and Ptbp1 knockdown cells. Data were presented as means +- SD. (F-H) Chromatin immunoprecipitation (ChIP) experiments with HNRNPK (F) or RNA polymerase II (G) antibodies; CHIRP experiment with specific anti- Lncenc1 probes (H). Relative enrichment of ChIP or CHIRP and input DNA were determined by qPCR. Primers were designed at promoters of glycolysis genes, pluripotency genes, and control genes ( B2m and Actb ), and two intergenic regions were used as negative controls. Data are shown as means +- SD of three independent experiments. Statistical significance of t test: * p < 0.05, ** p < 0.01, *** p < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 PTBP1 knockout embryos implant but are severely growth-retarded. The small category of embryos from a heterozygous intercross were identified by PCR and immunofluorescence as PTBP1 knockout embryos. A) PCR products for the stop cassette and PTBP1 intron 2 were separated on agarose gels and scored blindly. 5/5 small embryos were genotyped as homozygous. 15/17 large control embryos were genotyped as heterozygous or wild-type with 2 false negatives due to the less efficient intron PCR. B) Serial paraffin sections of E7.5 embryos were stained with hematoxylin and eosin (left column) or labelled with DAPI (middle column) and the PTBP1 antibody (right column). The top row shows a large embryo (in an oblique section) with strong nuclear PTBP1 staining of the embryo proper (dashed line) and the surrounding tissue. Both small embryos were characterised by a lack of the nuclear PTBP1 signal while showing nuclear PTBP1 in surrounding, most likely maternal cells. Interpretative diagrams and a quantification of the embryo section area are shown on the right.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- 3 Fig. EGFP upregulation in cell-free translation systems. (A, B) Cell-free translation systems using (A) RRL and (B) HeLa cell lysate. EGFP expression was detected by using (A) anti-GFP mouse monoclonal or (B) anti-GFP rabbit polyclonal antibodies and quantified by western blot analysis. EGFP expression levels were normalized to that of beta-actin. Fold induction of EGFP was calculated relative to the number of cells incubated with each SINEUP transcript: that is, transcribed from pCS2+ vector (pCS2+); IVT SINEUP; and modified with m 5 C, Psi, or N 1 mPsi. Data are shown as means +- SD of at least three independent experiments. * P < 0.05; ns, not significant (two-tailed Student's t -test). (C-E) SINEUP RBPs in cell-free lysates compared with HEK293T/17 cells. Images shown are representative western blots of (C) RBPs in the cell-free lysate compared with HEK293T/17 cell lysate. The protein levels of the SINEUP RBPs (D) PTBP1 and (E) HNRNPK were normalized to that of beta-actin. Fold change of protein levels is shown as the means +- SD of at least three independent experiments. *** P < 0.001, ns, not significant (two-tailed Student's t -test).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 10. Enhancement of UCHL1 by synthetic SINEUP-UCHL1. ( A ) Translational up-regulation of UCHL1 by transfection of SINEUP-UCHL1 expression vectors. Representative Western blotting image on the UCHL1 protein level (top) and quantification of the UCHL1 level (bottom). ** P < 0.01, ns: not significant, by Student's t -test. Data are means +- SD from at least 3 independent experiments. ( B ) Quantification of the UCHL1 mRNA and SINEUP RNA levels following transfection with SINEUP expression vectors. Data are means +- SD from at least three independent experiments. ( C ) Subcellular localization of SINEUP RNAs and Uchl1 mRNAs. Bars indicate 5 mum. ( D ) Quantitative comparison of co-localization of Uchl1 mRNAs and SINEUP RNAs in the cytoplasm. ** P < 0.01 by Student's t -test. Data are means +- SD of more than 10 individual cell images. ( E ) Translational up-regulation of UCHL1 by transfection with SINEUP-UCHL1 expression vectors. Representative Western blotting images of knockdown (KD) of PTBP1 and HNRNPK mediated by siRNA_PTBP1 (a1,2) and siRNA HNRNPK (b1,2), respectively. Numbers under the bottom row indicate knockdown efficiency compared with the cells transfected with SINEUP-UCHL1 and negative control siRNA (c1,2, SINEUP-UCHL1*). ( F ) Representative FISH images following knockdown (KD) of PTBP1 (e-h) or HNRNPK (i-l) by siRNAs. Bars indicate 5 mum. ( G ) Quantitative comparison of co-localization of Uchl1 mRNAs and SINEUP RNAs in the cytoplasm when PTBP1 (F, h) or HNRN
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4. Knockdown of SINEUP RBPs. ( A1, B1 ) Representative Western blotting images of knockdown (KD) of PTBP1 ( A1 ) and HNRNPK ( B1 ) mediated by siRNA-PTBP1 and siRNA-HNRNPK, respectively. Numbers under the bottom row indicate knockdown efficiency compared with cells co-transfected with SINEUP-GFP and negative control siRNA (SINEUP-GFP in C1 and C2 ). ( C1 ) Representative Western blotting images of transfection with negative control siRNA. ( A2, B2, C2 ) Protein levels of EGFP were quantified by Western blotting analysis. EGFP expression levels were normalized to that of beta-actin. Fold induction of EGFP was calculated relative to cells transfected with the siRNA indicated in the above panels A1, B1, or C1 respectively. ** P < 0.01, * P < 0.05; ns: not significant by Student's t -test. Data are means +- SD of at least three independent experiments. ( D ) Representative FISH images following knockdown (KD) of PTBP1 (f-j) or HNRNPK (k-o) by siRNAs, and negative control siRNA; siRNA_Cont. (a-e). Bars indicate 5 mum. ( E ) Quantitative comparison of co-localization of EGFP mRNAs and SINEUP RNAs in the cytoplasm when PTBP1 (D, j) or HNRNPK (D, o) were knocked down. ** P < 0.01 by Student's t -test. Data are means +- SD of 10 individual cell images. ( F ) Quantitative nuclear distribution of SINEUP-GFP RNAs following knockdown of PTBP1 (D, h) or HNRNPK (D, m) by siRNAs; the results are compared with the cells transfected negative control siRNA; siRNA_Cont (D, c). For both PT
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 6. Overexpression of SINEUP RBPs. ( A1 , B1 ) Representative Western blotting images comparing EGFP expression after overexpression of PTBP1 (+PTBP1) ( A1 ) or HNRNPK (+HNRNPK) ( B1 ) with that in the non-overexpressing control (Cont.). Numbers under the image show the overexpression efficiency compared with controls. ( A2 , A3 , B2 , B3 ) Quantification of EGFP levels after non-/overexpression of PTBP1 ( A2 , A3 ) or HNRNPK ( B2 , B3 ) when cells were transfected with EGFP vector alone ( A2 , B2 ) or co-transfected with EGFP and SINEUP-GFP vectors ( A3 , B3 ). * P < 0.05, ns: not significant by Student's t -test. Data are means +- SD of at least three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 7. Subcellular distribution of SINEUP RNAs after overexpression of SINEUP RBPs. ( A1 , B1 ) Representative RNA FISH with immunofluorescence images of the subcellular distribution of SINEUP RNAs and SINEUP RBPs in cells overexpressing PTBP1 (+PTBP1) ( A1 ) or HNRNPK (+HNRNPK) ( B1 ). Images for cells co-transfected with EGFP and SINEUP-GFP vectors (left images, A1 and B1 ) were compared with cells transfected with SINEUP-GFP vector alone (right images, A1 and B1 ). Bars indicate 5 mum. ( A2 , A3 , B2 , B3 ) Quantitative comparison of SINEUP-GFP RNA distribution between cells overexpressing PTBP1 ( A2 , A3 ) or HNRNPK ( B2 , B3 ) and cells without overexpression (Cont.). Results for cells co-transfected with EGFP and SINEUP-GFP vectors ( A2 and B2 ) and those transfected with SINEUP-GFP vector alone ( A3 , B3 ) are shown. SINEUP RNA signals were detected using Icy Spot Detector. The ratio of spots in the nucleus and the cytoplasm were compared between overexpression and non-overexpression of SINEUP RBPs. * P < 0.05 by Student's t -test. Data are means +- SD of at least 10 independent cell images.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 9. Identification of the SINEUP RBPs binding regions by seCLIP-seq analysis. ( A ) Read coverage along SINEUP-GFP shown by seCLIP with HNRNPK or PTBP1. Labeled boxes show the identified binding regions with HNRNPK ( - ) and PTBP1 ( - ) on SINEUP-GFP transcripts. ( B ) Sequences of binding sites of HNRNPK ( - ) and PTBP1 ( - ) on SINEUP-GFP transcripts shown in A. ( C ) Schematic representation of annealing sites (a), (b) and (c) with the SINEUP-GFP and SCR mutant are shown. ( D ) Representative Western blotting image on the EGFP level (top) and quantification of the EGFP level (bottom). EGFP vector and the mutants were co-transfected in HEK-293T/17. ** P < 0.01, ns: not significant, by Student's t -test. Data are means +- SD from at least three independent experiments. The SINEUP deletion mutants Delta - (deleted HNRNPK binding regions from SINEUP-GFP), and Delta - (deleted PTBP1 binding regions from SINEUP-GFP) were shown in A and B, and annealing sites are shown in C. ( E ) Quantification of EGFP mRNA and SINEUP RNA levels following co-transfection with EGFP and SINEUP expression vectors. Data are means +- SD from at least three independent experiments. The SINEUP deletion mutants Delta - (deleted HNRNPK binding regions from SINEUP-GFP), and Delta - (deleted PTBP1 binding regions from SINEUP-GFP) were shown in A and B, and annealing sites were shown in C. ns: not significant by Student's t -test. Data are means +- SD from at least three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 3 SMAR1 makes a triple complex with PTBP1 and HDAC6. a Expression of hnRNP A1, hnRNP A2 and PTBP1 upon shRNA-mediated knockdown of SMAR1 in MCF7. b Co-IP of SMAR1 with hnRNP A1, hnRNP A2, PTBP1, and HDAC6 in MCF7 suggest that SMAR1 interacts with PTBP1 and HDAC6. c Co-IP of PTBP1 with SMAR1, HDAC1, and HDAC6 in MCF7 suggest that PTBP1 interacts with SMAR1 and HDAC6. d Co-IP of HADC6 with hnRNP A1, hnRNP A2, PTBP1, and SMAR1 in MCF7 confirms that HDAC6 interacts with PTBP1 and SMAR1. e Sequential Co-IP of SMAR1 with HDAC6 and PTBP1 in MCF7 suggest that SMAR1-HDAC6 makes a triple complex with PTBP1
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 3. Polypyrimidine tract-binding protein 1 (PTBP1) protein binds to Nanos3 mRNA. (A) Fragments per kilobase of exon per million mapped reads (FPKM) values of Ptbp1 and Nanos3 are shown in the left and right panels, respectively. (B) The reduction of Nanos3 mRNA expression in Ptbp1 conditional knockout germline stem cells (GSCs) was also confirmed by quantitative real-time PCR. Asterisks indicate a significant difference (** P < 0.01; n = 3). (C) Immunoblotting of PTBP1 (upper panel) or pan-ACTIN (bottom panel) following immunoprecipitation by an anti-PTBP1 antibody or IgG-conjugated microbeads using wild-type GSC lysate. (D) mRNA was extracted from anti-PTBP1-immunoprecipitated GSC lysate and analyzed by reverse transcription-PCR using a Nanos3 -specific primer set.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- 10.1371/journal.pone.0271453.g002 Fig 2 RNA affinity chromatography identifies PTBP1 and other SELENOP 3' UTR binding proteins. A) Diagram of the components used for glutathione agarose affinity chromatography and RNA constructs used. B) SDS PAGE analysis of rat liver proteins eluted from a GST-MS2 column bound with either control RNA, SELENOP 3' UTR (FL 3' UTR), and the interSECIS deletion mutant (DeltaInter). The gel was stained with Coomassie and the bands of interest excised from the gel for LC MS/MS analysis. C) SDS PAGE and D) immunoblot analysis of proteins eluted from RNA affinity experiments as described in A) except that HepG2 lysate was used. The immunoblot was probed with anti-PTBP1 antibody. E) Peptide counts and cognate genes that were identified by LC MS/MS MudPIT analysis after RNA chromatography using control RNA (Cntl), wild-type SELENOP 3' UTR (WT) and an RNA lacking the first 273 nt of the SELENOP 3' UTR (Delta273). The ratio of peptide counts from WT UTR versus control RNA is shown (WT:Cntr). The genes shown here correspond to those with a ratio of 5 or above and a peptide count of 10 or above.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4. Sequence motif enrichments reveal a role for PTB in regulating SMC CEs. ( A ) Motif enrichment analysis. The diagram indicates k-mer and RNA-compete motifs enriched in the indicated regions associated with differentiated (top) or proliferative (bottom) exons. All motifs were significantly enriched ( P < 0.01). The five motifs marked with an asterisk also passed a FDR test < 0.05. Numbers adjacent to motifs indicate log 2 fold enrichment. ( B ) PTBP1 and PTBP2 were knockdown in rat PAC1 cells and its effects assessed in SMC CE from Figure 2 . ( C ) Effect of PTBP1/2 knockdown in rat PAC1 cells on Atp2b4 minigene, containing the regulated exon with 225 nt of upstream intron and 275 nt of downstream intron, cloned into an GFP exon trapping vector. ( D ) Effect of overexpression of STAR family proteins on Atp2b4 minigene in rat PAC1 cells. Histograms show mean and standard deviation of the mean of at least three samples. Statistically significance was calculated using Student's t -test, and is shown * P < 0.05, ** P < 0.01, *** P < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 EMCV IRES inhibited the expression of transgene on the translational level. ( A ) HEK293 cells were transduced with rAAV6-CMVp- gfp at 10,000 vgs/cell. The relative rAAV6 genome content was detected by qPCR using GFP primers and ITR primers. ( B ) K562 and ( C ) HEK293 cells were transduced with rAAV6-CMVp- gfp and rAAV6-CMVp-EMCV IRES- gfp at 10,000 vgs/cell. Total DNA and RNA were isolated at 4 days post-transduction for qPCR. Transgene expression was detected by fluorescence microscopy at 72 hours post-transduction. (D) Western blot of total cell extracts (lysate) from HEK293 cells and K562 cells after rAAV6-CMVp- gfp or rAAV6-CMVp-EMCV IRES- gfp infection for Gemin5, PTBP1, and PCBP2 expression.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 4 Polypyrimidine tract-binding protein 1 (PTBP1) physically interacts with pre-let-7a-1/7d. a Predicated PTBP1-binding sites in the human pre-let-7a-1/7d using RBPmap ( http://rbpmap.technion.ac.il ). The position, genomic motifs, occurrence, z -score, and P -value were shown. b RNA pull-down followed by western blotting. Either cell lysates of Hepa1 or Huh7 or cell lysates prepared from Hepa1 or Huh7 cells transfected without (-) or with (+) H19 overexpression plasmid were incubated with biotin-labeled pre-let-7d (sense or antisense) or pre-let-7a-1 (sense or antisense) probes. After pull down, the recruited PTBP1 to probes was examined by western blotting with anti-PTBP1 antibody. c RNA-immunoprecipitation (RIP) assay to detect the interactions between PTBP1 and pre-let-7a-1 or pre-let-7d using anti-PTBP1 or immunoglobulin GIgG (negative control) in Huh7 cells. Data were shown as mean +- SEM from triplicate assays. *** P < 0.001
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 5 H19 decreases polypyrimidine tract-binding protein 1 (PTBP1) expression in cholestasis. ( a ) Quantitative PCR of PTBP1 expression in control and H19-overexpression or maternal H19 knockout bile duct ligation (BDL) mouse livers. Left, -BDL vs H19-BDL; right, Con-BDL vs HKO-BDL. Data were shown as mean +- SEM from triplicate assays ( n = 5 mice/group). *** P < 0.001. b Western blotting of PTBP1 in mouse livers as a . c , d Western blotting of the expression of indicated proteins in cytoplasm and nucleus fractions prepared from BDL mouse livers. Cyclophilin A was used as a cytoplasmic fraction maker and Lamin A/C was used as a nuclear fraction marker. c , d Protein samples were pooled from five individual mice per group
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation