MA3-067
antibody from Invitrogen Antibodies
Targeting: RAB9A
RAB9
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Immunoelectron microscopy
Immunoelectron microscopy Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [21]
- Comments [0]
- Validations
- Western blot [3]
- Immunocytochemistry [4]
- Immunohistochemistry [3]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-067 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- RAB9 Monoclonal Antibody (Mab9)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- MA3-067 recognizes prenylated and non-prenylated rab 9 from canine, human, monkey, rat, hamster and bovine tissues. This antibody does not cross-react with other rab family members. MA3-067 has been successfully used in Western blot, immunofluorescence, EM immunocytochemistry and immunoprecipitation procedures. By Western blot, this antibody detects an ~26 kDa protein representing rab 9 from K562 cell extract. The MA3-067 antigen is recombinant canine rab 9a.
- Reactivity
- Human, Rat, Bovine, Canine, Hamster
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- Mab9
- Vial size
- 100 µL
- Concentration
- 1 mg/mL
- Storage
- -20° C, Avoid Freeze/Thaw Cycles
Submitted references Herpes Simplex Virus Entry by a Nonconventional Endocytic Pathway.
Endosomal trafficking defects in patient cells with KIAA1109 biallelic variants.
Endocytosis of Activated Muscarinic m2 Receptor (m2R) in Live Mouse Hippocampal Neurons Occurs via a Clathrin-Dependent Pathway.
Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons.
Vesicular acetylcholine transporter (VAChT) over-expression induces major modifications of striatal cholinergic interneuron morphology and function.
BAIAP3, a C2 domain-containing Munc13 protein, controls the fate of dense-core vesicles in neuroendocrine cells.
Dynamic internalization and recycling of a metal ion transporter: Cu homeostasis and CTR1, the human Cu⁺ uptake system.
Microvillus inclusion disease: loss of Myosin vb disrupts intracellular traffic and cell polarity.
Lipid droplet-binding protein TIP47 regulates hepatitis C Virus RNA replication through interaction with the viral NS5A protein.
Affibody-mediated retention of the epidermal growth factor receptor in the secretory compartments leads to inhibition of phosphorylation in the kinase domain.
Antibody-based proteomics for esophageal cancer: Identification of proteins in the nuclear factor-kappaB pathway and mitotic checkpoint.
Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein.
High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy.
Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens.
Activation of cellular Arf GTPases by poliovirus protein 3CD correlates with virus replication.
Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment.
Nitric oxide-dependent processing of heparan sulfate in recycling S-nitrosylated glypican-1 takes place in caveolin-1-containing endosomes.
Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments.
Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides.
Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides.
Rab GDI: a solubilizing and recycling factor for rab9 protein.
Tebaldi G, Pritchard SM, Nicola AV
Journal of virology 2020 Nov 23;94(24)
Journal of virology 2020 Nov 23;94(24)
Endosomal trafficking defects in patient cells with KIAA1109 biallelic variants.
Kane MS, Diamonstein CJ, Hauser N, Deeken JF, Niederhuber JE, Vilboux T
Genes & diseases 2019 Mar;6(1):56-67
Genes & diseases 2019 Mar;6(1):56-67
Endocytosis of Activated Muscarinic m2 Receptor (m2R) in Live Mouse Hippocampal Neurons Occurs via a Clathrin-Dependent Pathway.
Lambert L, Dubayle D, Fafouri A, Herzog E, Csaba Z, Dournaud P, El Mestikawy S, Bernard V
Frontiers in cellular neuroscience 2018;12:450
Frontiers in cellular neuroscience 2018;12:450
Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons.
Cheng XT, Xie YX, Zhou B, Huang N, Farfel-Becker T, Sheng ZH
The Journal of cell biology 2018 Sep 3;217(9):3127-3139
The Journal of cell biology 2018 Sep 3;217(9):3127-3139
Vesicular acetylcholine transporter (VAChT) over-expression induces major modifications of striatal cholinergic interneuron morphology and function.
Janickova H, Prado VF, Prado MAM, El Mestikawy S, Bernard V
Journal of neurochemistry 2017 Sep;142(6):857-875
Journal of neurochemistry 2017 Sep;142(6):857-875
BAIAP3, a C2 domain-containing Munc13 protein, controls the fate of dense-core vesicles in neuroendocrine cells.
Zhang X, Jiang S, Mitok KA, Li L, Attie AD, Martin TFJ
The Journal of cell biology 2017 Jul 3;216(7):2151-2166
The Journal of cell biology 2017 Jul 3;216(7):2151-2166
Dynamic internalization and recycling of a metal ion transporter: Cu homeostasis and CTR1, the human Cu⁺ uptake system.
Clifford RJ, Maryon EB, Kaplan JH
Journal of cell science 2016 Apr 15;129(8):1711-21
Journal of cell science 2016 Apr 15;129(8):1711-21
Microvillus inclusion disease: loss of Myosin vb disrupts intracellular traffic and cell polarity.
Thoeni CE, Vogel GF, Tancevski I, Geley S, Lechner S, Pfaller K, Hess MW, Müller T, Janecke AR, Avitzur Y, Muise A, Cutz E, Huber LA
Traffic (Copenhagen, Denmark) 2014 Jan;15(1):22-42
Traffic (Copenhagen, Denmark) 2014 Jan;15(1):22-42
Lipid droplet-binding protein TIP47 regulates hepatitis C Virus RNA replication through interaction with the viral NS5A protein.
Vogt DA, Camus G, Herker E, Webster BR, Tsou CL, Greene WC, Yen TS, Ott M
PLoS pathogens 2013;9(4):e1003302
PLoS pathogens 2013;9(4):e1003302
Affibody-mediated retention of the epidermal growth factor receptor in the secretory compartments leads to inhibition of phosphorylation in the kinase domain.
Vernet E, Lundberg E, Friedman M, Rigamonti N, Klausing S, Nygren PA, Gräslund T
New biotechnology 2009 Sep;25(6):417-23
New biotechnology 2009 Sep;25(6):417-23
Antibody-based proteomics for esophageal cancer: Identification of proteins in the nuclear factor-kappaB pathway and mitotic checkpoint.
Uemura N, Nakanishi Y, Kato H, Nagino M, Hirohashi S, Kondo T
Cancer science 2009 Sep;100(9):1612-22
Cancer science 2009 Sep;100(9):1612-22
Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein.
Cirillo C, Sarnelli G, Esposito G, Grosso M, Petruzzelli R, Izzo P, Calì G, D'Armiento FP, Rocco A, Nardone G, Iuvone T, Steardo L, Cuomo R
Neurogastroenterology and motility 2009 Nov;21(11):1209-e112
Neurogastroenterology and motility 2009 Nov;21(11):1209-e112
High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy.
Treré D, Brighenti E, Donati G, Ceccarelli C, Santini D, Taffurelli M, Montanaro L, Derenzini M
Annals of oncology : official journal of the European Society for Medical Oncology 2009 Nov;20(11):1818-23
Annals of oncology : official journal of the European Society for Medical Oncology 2009 Nov;20(11):1818-23
Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens.
Rögelsperger O, Ekmekcioglu C, Jäger W, Klimpfinger M, Königsberg R, Krenbek D, Sellner F, Thalhammer T
Journal of pineal research 2009 May;46(4):422-32
Journal of pineal research 2009 May;46(4):422-32
Activation of cellular Arf GTPases by poliovirus protein 3CD correlates with virus replication.
Belov GA, Habbersett C, Franco D, Ehrenfeld E
Journal of virology 2007 Sep;81(17):9259-67
Journal of virology 2007 Sep;81(17):9259-67
Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment.
Ivanov AI, Nusrat A, Parkos CA
Molecular biology of the cell 2004 Jan;15(1):176-88
Molecular biology of the cell 2004 Jan;15(1):176-88
Nitric oxide-dependent processing of heparan sulfate in recycling S-nitrosylated glypican-1 takes place in caveolin-1-containing endosomes.
Cheng F, Mani K, van den Born J, Ding K, Belting M, Fransson LA
The Journal of biological chemistry 2002 Nov 15;277(46):44431-9
The Journal of biological chemistry 2002 Nov 15;277(46):44431-9
Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments.
Lorenzo ME, Jung JU, Ploegh HL
Journal of virology 2002 Jun;76(11):5522-31
Journal of virology 2002 Jun;76(11):5522-31
Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides.
Shapiro AD, Pfeffer SR
The Journal of biological chemistry 1995 May 12;270(19):11085-90
The Journal of biological chemistry 1995 May 12;270(19):11085-90
Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides.
Shapiro AD, Pfeffer SR
The Journal of biological chemistry 1995 May 12;270(19):11085-90
The Journal of biological chemistry 1995 May 12;270(19):11085-90
Rab GDI: a solubilizing and recycling factor for rab9 protein.
Soldati T, Riederer MA, Pfeffer SR
Molecular biology of the cell 1993 Apr;4(4):425-34
Molecular biology of the cell 1993 Apr;4(4):425-34
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of RAB9 was performed by loading 25 µg of K562 (lane 1), 293 (lane 2) and Hela (lane 3) onto an SDS polyacrylamide gel. Proteins were transferred to a PVDF membrane and blocked at 4ºC overnight. The membrane was probed with a RAB9 monoclonal antibody (Product # MA3-067) at a dilution of 1:2000 overnight at 4°C, washed in TBST, and probed with an HRP-conjugated secondary antibody for 1 hr at room temperature in the dark. Chemiluminescent detection was performed using Pierce ECL Plus Western Blotting Substrate (Product # 32132). Images were taken at an exposure time of 4 min (lane 1) and 2 min (lanes 2 and 3). Results show a band at ~23 kDa.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
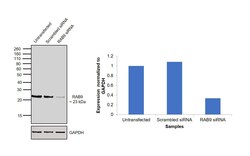
- Experimental details
- Knockdown of RAB9 was achieved by transfecting HEK-293 cells with RAB9A specific siRNAs (Silencer® select Product # s17916, s17917). Western blot analysis (Fig. a) was performed using membrane enriched extracts from the HEK-293 knockdown cells (lane 3), non-specific scrambled siRNA transfected cells (lane 2) and untransfected cells (lane 1). The blots were probed with RAB9 Mouse Monoclonal Antibody (Product # MA3-067, 1:2500 dilution) and Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000 dilution). Densitometric analysis of this western blot is shown in histogram (Fig. b). Decrease in signal upon siRNA mediated knock down confirms that antibody is specific to RAB9A.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot was performed using Anti-RAB9 Mouse Monoclonal Antibody (Product # MA3-067) and a 23 kDa band corresponding to RAB9 was observed in cell lines tested. Membrane enriched extracts (30 µg lysate) of HeLa (Lane 1), HEK-293 (Lane 2) and MCF7 (Lane 3) were electrophoresed using NuPAGE™ 12% Bis-Tris Protein Gel (Product # NP0341BOX). Resolved proteins were then transferred onto a nitrocellulose membrane (Product # IB23001) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (1:2000 dilution) and detected by chemiluminescence with Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000 dilution) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescent analysis of RAB9 using RAB9 Monoclonal Antibody (Mab9) (Product # MA3-067) shows staining in A2058 Cells. RAB9 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing RAB9 (Product # MA3-067) at a dilution of 1:100 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescent analysis of RAB9 using RAB9 Monoclonal Antibody (Mab9) (Product # MA3-067) shows staining in Hela Cells. RAB9 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing RAB9 (Product # MA3-067) at a dilution of 1:200 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescent analysis of RAB9 using RAB9 Monoclonal Antibody (Mab9) (Product # MA3-067) shows staining in U251 Cells. RAB9 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing RAB9 (Product # MA3-067) at a dilution of 1:100 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescence analysis of RAB9 was performed using HEK-293 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with RAB9 Mouse Monoclonal Antibody (Product # MA3-067) at 1:200 dilution in 0.1% BSA and incubated overnight at 4 degree and then labeled with Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32723) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green) in HEK-293 cells. Nuclei (Panel b: blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image of HEK-293 cells showing cytoplasmic (Golgi, ER, endosome pattern) and plasma membrane localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemistry was performed on cancer biopsies of deparaffinized Human prostate carcinoma tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing RAB9 (Product # MA3-067) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human stomach tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing RAB9 (Product # MA3-067) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human tonsil tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing RAB9 (Product # MA3-067) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 2 Analysis of endosomal pathway at steady state in fibroblasts. Immunostaining for actin (magenta), lysosomal protein LAMP3 (red), and early endosome protein EEA1 (green) in patient B (Pt. B) and control (PCS Ctl) cells (A i-ii). Immunofluorescence imaging of patient B (Pt. B) and control (PCS Ctl) cells stained for actin (magenta), late endosome maker Rab9 (red), and early endosome protein EEA1 (green) (A iii-iv). Quantification of the average number of early endosomes (p = 8.49E-8) (B), lysosomes (p = 7.09E-28) (C), and late endosomes (not significantly different) (D) in fixed cell images from Pt. B and PCS Ctl cells. DNA counterstaining in blue (Hoechst). Error bars represent SEM. ** p < 0.01 * = p < 0.025. Fig. 2
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 4 Dextran endocytosis trafficking assay. Dextran overall intensity within the cell at 5 and 10 min of dextran chase in patient B (Pt. B) and control (PCS Ctl) cells (A). Correlation of dextran signal with EEA1 signal in cells at 5 and 10 min of dextran chase in Pt. B and PCS Ctl (B). Representative micrographs of immunofluorescence staining of dextran (green) labeled Pt. B and PCS Ctl cells at 5 min with various endosomal markers: Rab9 late endosomes (red) and EEA1 early endosomes (magenta) (C i-ii), Rab11 fast- (red) and Rab4 slow-recycling endosomes (C iii-iv), and Rab9 late endosomes (red) with Rab7 lysosomal targeted vesicles (magenta) (C v-vi). DNA counterstaining in blue (Hoeschst) (C). Correlation of each endosomal and trafficking marker with dextran signal at 0, 5 and 10 min chase time points (t0, t5 and t10, respectively) in dextran assay is shown as indicated in the vertical axis label (D-G). Error bars represent SEM. ** = p < 0.01, * = p < 0.025. Fig. 4
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- FIGURE 9 Immunohistochemical localization of m2R in neuronal compartments involved in endocytosis, synthesis, maturation, and degradation in fixed hippocampal neurons. Hippocampal neurons were transfected with m2R-WT. Neurons were stimulated with CCh at 30 muM for 6, 20 min and 1 h fixed, and processed for visualization of m2R together with markers of intraneuronal compartments and observed by confocal microscopy. ( A-C) 6 min after CCh stimulation (30 muM), some m2R immunopositive punta colocalize with CHC in clathrin-coated pits, EEA1 in early endosomes and Rab9 in late endosomes (arrow heads). (D-E) Twenty minutes after CCh stimulation (30 muM), we failed to detect no colocalization of m2R with PDI, a marker of endoplasmic reticulum and GM130, a marker of Golgi apparatus. (F-F) One hour after CCh stimulation (30 muM), some m2R immunopositive puncta colocalize with CathD, a marker of lysosomes (arrow heads). The quantitative analysis of the colocalization of m2R and markers of subcellular compartment in neurons was performed using the Jacop Plugin of ImageJ and statistical data are reported from the Costes's randomization-based colocalization module (see Materials and Methods). Data are expressed as a Pearson's coefficient (pc) and pc were compared using the Mann-Whitney U -test. Our analysis shows that the colocalization of the immunofluorescent signals for m2R with CHC, EEA1, Rab9, and CathD is higher after treatment with CCh compared to untreated neurons (CHC, Rab9
 Explore
Explore Validate
Validate Learn
Learn