Antibody data
- Antibody Data
- Antigen structure
- References [29]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [28]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 47-0319-42 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD31 (PECAM-1) Monoclonal Antibody (WM-59 (WM59)), APC-eFluor™ 780, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The WM59 monoclonal antibody reacts with human CD31, also known as platelet-endothelial cell adhesion molecule-1 (PECAM-1) and gpIIa. This 130-140 kDa surface protein is expressed by endothelial cells and at low levels on leukocytes and platelets. It has been reported that CD38 binds to CD31. Homotypic interaction of CD31 is important in adhesion, cell-cell and cell-matrix interaction, and signal transduction.
- Antibody clone number
- WM-59 (WM59)
- Concentration
- 5 µL/Test
Submitted references Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs.
Expression and role of interleukin-1β and associated biomarkers in deep vein thrombosis.
Down-Regulated Exosomal MicroRNA-221 - 3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair.
Developing Human Skin Contains Lymphocytes Demonstrating a Memory Signature.
An Isoform of the Oncogenic Splice Variant AIMP2-DX2 Detected by a Novel Monoclonal Antibody.
PF-127 hydrogel plus sodium ascorbyl phosphate improves Wharton's jelly mesenchymal stem cell-mediated skin wound healing in mice.
Deep Mining of Complex Antibody Phage Pools Generated by Cell Panning Enables Discovery of Rare Antibodies Binding New Targets and Epitopes.
Expansion of functional personalized cells with specific transgene combinations.
Prostaglandin E(2) Is Required for BMP4-Induced Mesoderm Differentiation of Human Embryonic Stem Cells.
Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells.
Distinctive expression of T cell guiding molecules in human autoimmune lymph node stromal cells upon TLR3 triggering.
Impaired lymph node stromal cell function during the earliest phases of rheumatoid arthritis.
Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations.
Increased infiltration and tolerised antigen-specific CD8(+) T(EM) cells in tumor but not peripheral blood have no impact on survival of HCMV(+) glioblastoma patients.
Targeted Disruption of TCF12 Reveals HEB as Essential in Human Mesodermal Specification and Hematopoiesis.
MMP-2 and MMP-14 Silencing Inhibits VEGFR2 Cleavage and Induces the Differentiation of Porcine Adipose-Derived Mesenchymal Stem Cells to Endothelial Cells.
ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells.
TGF-β reduces DNA ds-break repair mechanisms to heighten genetic diversity and adaptability of CD44+/CD24- cancer cells.
CXCL12/CXCR4 activation by cancer-associated fibroblasts promotes integrin β1 clustering and invasiveness in gastric cancer.
Human esophageal myofibroblasts secrete proinflammatory cytokines in response to acid and Toll-like receptor 4 ligands.
Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis.
Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes.
Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta.
Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature.
Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer.
Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells.
Cells expressing the C/EBPbeta isoform, LIP, engulf their neighbors.
Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression.
Isolation of an adult blood-derived progenitor cell population capable of differentiation into angiogenic, myocardial and neural lineages.
Li Y, Chen X, Jin R, Chen L, Dang M, Cao H, Dong Y, Cai B, Bai G, Gooding JJ, Liu S, Zou D, Zhang Z, Yang C
Science advances 2021 Feb;7(9)
Science advances 2021 Feb;7(9)
Expression and role of interleukin-1β and associated biomarkers in deep vein thrombosis.
Pai RZ, Fang Q, Tian G, Zhu B, Ge X
Experimental and therapeutic medicine 2021 Dec;22(6):1366
Experimental and therapeutic medicine 2021 Dec;22(6):1366
Down-Regulated Exosomal MicroRNA-221 - 3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair.
Sun L, Zhu W, Zhao P, Zhang J, Lu Y, Zhu Y, Zhao W, Liu Y, Chen Q, Zhang F
Frontiers in cell and developmental biology 2020;8:263
Frontiers in cell and developmental biology 2020;8:263
Developing Human Skin Contains Lymphocytes Demonstrating a Memory Signature.
Dhariwala MO, Karthikeyan D, Vasquez KS, Farhat S, Weckel A, Taravati K, Leitner EG, Clancy S, Pauli M, Piper ML, Cohen JN, Ashouri JF, Lowe MM, Rosenblum MD, Scharschmidt TC
Cell reports. Medicine 2020 Nov 17;1(8):100132
Cell reports. Medicine 2020 Nov 17;1(8):100132
An Isoform of the Oncogenic Splice Variant AIMP2-DX2 Detected by a Novel Monoclonal Antibody.
Kim DG, Nguyen TTH, Kwon NH, Sung J, Lim S, Kang EJ, Lee J, Seo WY, Kim A, Chang YS, Shim H, Kim S
Biomolecules 2020 May 27;10(6)
Biomolecules 2020 May 27;10(6)
PF-127 hydrogel plus sodium ascorbyl phosphate improves Wharton's jelly mesenchymal stem cell-mediated skin wound healing in mice.
Deng Q, Huang S, Wen J, Jiao Y, Su X, Shi G, Huang J
Stem cell research & therapy 2020 Apr 3;11(1):143
Stem cell research & therapy 2020 Apr 3;11(1):143
Deep Mining of Complex Antibody Phage Pools Generated by Cell Panning Enables Discovery of Rare Antibodies Binding New Targets and Epitopes.
Ljungars A, Svensson C, Carlsson A, Birgersson E, Tornberg UC, Frendéus B, Ohlin M, Mattsson M
Frontiers in pharmacology 2019;10:847
Frontiers in pharmacology 2019;10:847
Expansion of functional personalized cells with specific transgene combinations.
Lipps C, Klein F, Wahlicht T, Seiffert V, Butueva M, Zauers J, Truschel T, Luckner M, Köster M, MacLeod R, Pezoldt J, Hühn J, Yuan Q, Müller PP, Kempf H, Zweigerdt R, Dittrich-Breiholz O, Pufe T, Beckmann R, Drescher W, Riancho J, Sañudo C, Korff T, Opalka B, Rebmann V, Göthert JR, Alves PM, Ott M, Schucht R, Hauser H, Wirth D, May T
Nature communications 2018 Mar 8;9(1):994
Nature communications 2018 Mar 8;9(1):994
Prostaglandin E(2) Is Required for BMP4-Induced Mesoderm Differentiation of Human Embryonic Stem Cells.
Zhang B, He L, Liu Y, Zhang J, Zeng Q, Wang S, Fan Z, Fang F, Chen L, Lv Y, Xi J, Yue W, Li Y, Pei X
Stem cell reports 2018 Mar 13;10(3):905-919
Stem cell reports 2018 Mar 13;10(3):905-919
Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells.
Mieczkowska A, Schumacher A, Filipowicz N, Wardowska A, Zieliński M, Madanecki P, Nowicka E, Langa P, Deptuła M, Zieliński J, Kondej K, Renkielska A, Buckley PG, Crossman DK, Crowley MR, Czupryn A, Mucha P, Sachadyn P, Janus Ł, Skowron P, Rodziewicz-Motowidło S, Cichorek M, Pikuła M, Piotrowski A
Scientific reports 2018 Jul 27;8(1):11339
Scientific reports 2018 Jul 27;8(1):11339
Distinctive expression of T cell guiding molecules in human autoimmune lymph node stromal cells upon TLR3 triggering.
Hähnlein JS, Ramwadhdoebe TH, Semmelink JF, Choi IY, Berger FH, Maas M, Gerlag DM, Tak PP, Geijtenbeek TBH, van Baarsen LGM
Scientific reports 2018 Jan 29;8(1):1736
Scientific reports 2018 Jan 29;8(1):1736
Impaired lymph node stromal cell function during the earliest phases of rheumatoid arthritis.
Hähnlein JS, Nadafi R, de Jong T, Ramwadhdoebe TH, Semmelink JF, Maijer KI, Zijlstra IA, Maas M, Gerlag DM, Geijtenbeek TBH, Tak PP, Mebius RE, van Baarsen LGM
Arthritis research & therapy 2018 Feb 26;20(1):35
Arthritis research & therapy 2018 Feb 26;20(1):35
Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations.
Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, Watt FM
The Journal of investigative dermatology 2018 Apr;138(4):811-825
The Journal of investigative dermatology 2018 Apr;138(4):811-825
Increased infiltration and tolerised antigen-specific CD8(+) T(EM) cells in tumor but not peripheral blood have no impact on survival of HCMV(+) glioblastoma patients.
Bahador M, Gras Navarro A, Rahman MA, Dominguez-Valentin M, Sarowar S, Ulvestad E, Njølstad G, Lie SA, Kristoffersen EK, Bratland E, Chekenya M
Oncoimmunology 2017;6(8):e1336272
Oncoimmunology 2017;6(8):e1336272
Targeted Disruption of TCF12 Reveals HEB as Essential in Human Mesodermal Specification and Hematopoiesis.
Li Y, Brauer PM, Singh J, Xhiku S, Yoganathan K, Zúñiga-Pflücker JC, Anderson MK
Stem cell reports 2017 Sep 12;9(3):779-795
Stem cell reports 2017 Sep 12;9(3):779-795
MMP-2 and MMP-14 Silencing Inhibits VEGFR2 Cleavage and Induces the Differentiation of Porcine Adipose-Derived Mesenchymal Stem Cells to Endothelial Cells.
Almalki SG, Llamas Valle Y, Agrawal DK
Stem cells translational medicine 2017 May;6(5):1385-1398
Stem cells translational medicine 2017 May;6(5):1385-1398
ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells.
Almalki SG, Agrawal DK
Stem cell research & therapy 2017 May 12;8(1):113
Stem cell research & therapy 2017 May 12;8(1):113
TGF-β reduces DNA ds-break repair mechanisms to heighten genetic diversity and adaptability of CD44+/CD24- cancer cells.
Pal D, Pertot A, Shirole NH, Yao Z, Anaparthy N, Garvin T, Cox H, Chang K, Rollins F, Kendall J, Edwards L, Singh VA, Stone GC, Schatz MC, Hicks J, Hannon GJ, Sordella R
eLife 2017 Jan 16;6
eLife 2017 Jan 16;6
CXCL12/CXCR4 activation by cancer-associated fibroblasts promotes integrin β1 clustering and invasiveness in gastric cancer.
Izumi D, Ishimoto T, Miyake K, Sugihara H, Eto K, Sawayama H, Yasuda T, Kiyozumi Y, Kaida T, Kurashige J, Imamura Y, Hiyoshi Y, Iwatsuki M, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Watanabe M, Takamori H, Araki N, Tan P, Baba H
International journal of cancer 2016 Mar 1;138(5):1207-19
International journal of cancer 2016 Mar 1;138(5):1207-19
Human esophageal myofibroblasts secrete proinflammatory cytokines in response to acid and Toll-like receptor 4 ligands.
Gargus M, Niu C, Vallone JG, Binkley J, Rubin DC, Shaker A
American journal of physiology. Gastrointestinal and liver physiology 2015 Jun 1;308(11):G904-23
American journal of physiology. Gastrointestinal and liver physiology 2015 Jun 1;308(11):G904-23
Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis.
Yuan K, Orcholski ME, Panaroni C, Shuffle EM, Huang NF, Jiang X, Tian W, Vladar EK, Wang L, Nicolls MR, Wu JY, de Jesus Perez VA
The American journal of pathology 2015 Jan;185(1):69-84
The American journal of pathology 2015 Jan;185(1):69-84
Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes.
Brandau S, Jakob M, Bruderek K, Bootz F, Giebel B, Radtke S, Mauel K, Jäger M, Flohé SB, Lang S
PloS one 2014;9(9):e106903
PloS one 2014;9(9):e106903
Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta.
Dutertre CA, Clement M, Morvan M, Schäkel K, Castier Y, Alsac JM, Michel JB, Nicoletti A
PloS one 2014;9(2):e89983
PloS one 2014;9(2):e89983
Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature.
Park TS, Bhutto I, Zimmerlin L, Huo JS, Nagaria P, Miller D, Rufaihah AJ, Talbot C, Aguilar J, Grebe R, Merges C, Reijo-Pera R, Feldman RA, Rassool F, Cooke J, Lutty G, Zambidis ET
Circulation 2014 Jan 21;129(3):359-72
Circulation 2014 Jan 21;129(3):359-72
Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer.
Feng Y, Zhu M, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q, Zhang H, Levchenko A, Guerrero-Cazares H, Quiñones-Hinojosa A
Cell death & disease 2014 Dec 11;5(12):e1567
Cell death & disease 2014 Dec 11;5(12):e1567
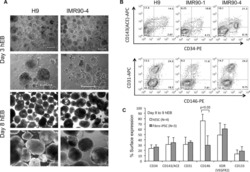
Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells.
Park TS, Zimmerlin L, Zambidis ET
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2013 Jan;83(1):114-26
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2013 Jan;83(1):114-26
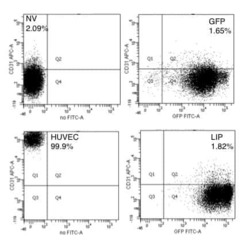
Cells expressing the C/EBPbeta isoform, LIP, engulf their neighbors.
Abreu M, Sealy L
PloS one 2012;7(7):e41807
PloS one 2012;7(7):e41807
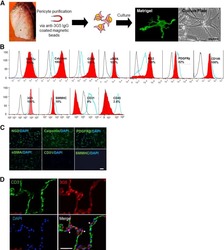
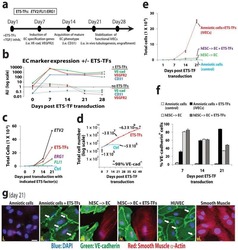
Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression.
Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, Schachterle W, Pulijaal VR, Mathew S, Chasen ST, Xiang J, Rosenwaks Z, Shido K, Elemento O, Rabbany SY, Rafii S
Cell 2012 Oct 26;151(3):559-75
Cell 2012 Oct 26;151(3):559-75
Isolation of an adult blood-derived progenitor cell population capable of differentiation into angiogenic, myocardial and neural lineages.
Porat Y, Porozov S, Belkin D, Shimoni D, Fisher Y, Belleli A, Czeiger D, Silverman WF, Belkin M, Battler A, Fulga V, Savion N
British journal of haematology 2006 Dec;135(5):703-14
British journal of haematology 2006 Dec;135(5):703-14
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
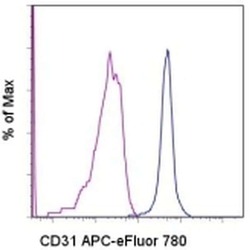
- Experimental details
- Staining of normal human peripheral blood cells with Mouse IgG1 K Isotype Control APC-eFluor® 780 (Product # 47-4714-82) (blue histogram) or Anti-Human CD31 (PECAM-1) APC-eFluor® 780 (purple histogram). Cells in the monocyte gate were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
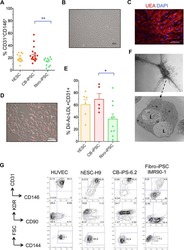
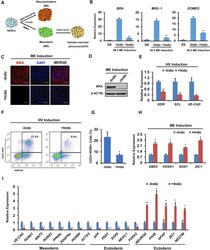
- Figure 2 De Novo Synthesis of PGE 2 Is Required for BMP4-Induced Mesoderm Specification of hESCs (A) Schematic of different induction methods. Indo, indomethacin. (B) qPCR analysis of mesodermal marker gene expression levels in hESCs with BMP4 induction plus DMSO (-Indo) or indomethacin (+Indo) for 48 hr. ** p < 0.01 compared with -Indo group. n >= 3 independent experiments. (C and D) Immunostaining (C) and western blotting (D) analysis for BRA in hESCs with BMP4 induction plus DMSO (-Indo) or indomethacin (+Indo) for 48 hr. Scale bars represent 50 mum. (E) hemato-vascular precursors (HV) marker gene expression levels in hESCs with BMP4 induction plus DMSO (-Indo) or indomethacin (+Indo) for 48 hr and hemato-vascular precursors induction (SFDM + 50 ng/mL VEGF + 50 ng/mL bFGF) for 4 days. * p < 0.05, ** p < 0.01 compared with -Indo group. n = 3 independent experiments. (F and G) Flow cytometry analysis of the percentage of KDR + CD31 + cells after hemato-vascular induction. * p < 0.05 compared with -Indo group. n = 3 independent experiments. (H) Neuroectoderm (NE) marker gene expression levels in hESCs with BMP4 induction plus DMSO (-Indo) or indomethacin (+Indo) for 48 hr. * p < 0.05 compared with -Indo group. n = 3 independent experiments. (I) qPCR analysis of tissue-specific gene expression levels in different engrafts. * p < 0.05, ** p < 0.01 compared with -Indo group. n = 3 independent experiments. Error bars indicate SD. See also Figure S2 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2--figure supplement 3. mRNA expression analysis of HDR genes in CD44-/CD24+ and CD44+/CD24- cells FACS sorted from cells lines and patient tumors. ( A ) Comparative expression of the indicated HDR genes in FACS-sorted CD44+/ CD24- cells relative to CD44-/ CD24+ cells from H1650, A549 and MCF7. mRNA expression was quantified by RT-qPCR. Each bar is the mean +- SD of three replicates from two different experiments and represents mRNA expression of the indicated gene. p-value *
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
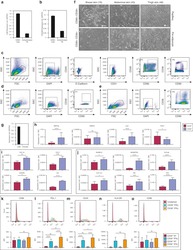
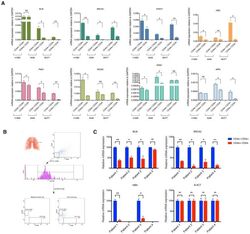
- Fig. 3 ATR2 siRNA transfection and immunophenotyping for EC markers. I Concentration selection for siRNA transfection. Three different concentrations (10, 35, and 50 nM) of ATR2 siRNA were used according to the manufacturer's protocol. Western blot analysis showed inhibition of ATR2 by 10, 35, and 50 nM of ATR2 siRNA. However, 50 nM of ATR2 siRNA showed the highest inhibition among all three different concentrations ( A ). ATR2 silencing by siRNA transfection with EGM compared with AMSCs with EGM and EGM + scrambled siRNA (negative control) ( B ). GAPDH was used as a housekeeping gene. II Flow cytometric analysis of PECAM1 (CD31) in four different groups; control group with EGM ( A ), AMSCs with EGM and MMP-2 siRNA ( B ), AMSCs with EGM and MMP-14 siRNA ( C ), and HUVECs as the positive control ( D ). Cell transfection with 5 muM of ATR2 siRNA for EGM ( E ), AMSCs with EGM and MMP-2 siRNA ( F ), and AMSCs with EGM and MMP-14 siRNA ( G ). Flow cytometry data were analyzed to show the significant differences between the groups ( H ). III Flow cytometric analysis of VE-cadherin (CD144) in four different groups: control group AMSCs with EGM ( A ), AMSCs with EGM and MMP-2 siRNA ( B ), AMSCs with EGM and MMP-14 siRNA ( C ), and HUVECs as the positive control ( D ). Cell transfection with 5 muM of ATR2 siRNA for EGM ( E ), AMSCs with EGM and MMP-2 siRNA ( F ), and AMSCs with EGM and MMP-14 siRNA ( G ). Flow cytometry data were analyzed to show the significant differences betwe
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
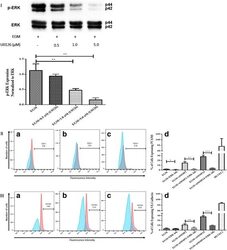
- Fig. 5 Inhibition of ERK phosphorylation and immunophenotyping for EC markers. I Concentration -dependent effect of ERK inhibitor (U0126). Three different concentrations (0.5, 1.0, and 5.0 muM) of U0126 were used. Western blot analysis showed significant inhibition of p-ERK by 1.0 and 5.0 muM of U0126. However, 5.0 muM of U0126 showed the highest inhibition among all three different concentrations. Phospho-ERK was normalized to its total protein expression. II Flow cytometric analysis of PECAM1 (CD31) with ERK inhibitor (U0126). Three different groups treated with 5.0 muM of U0126: AMSCs with EGM ( A ), AMSCs with EGM and MMP-2 siRNA ( B ), and AMSCs with EGM and MMP-14 siRNA ( C ). Flow cytometry data were analyzed to show the significant differences between the groups ( D ). III Flow cytometric analysis of VE-cadherin (CD144) with ERK inhibitor (U0126). Three different groups were treated with 5.0 muM of U0126: AMSCs with EGM ( A ), AMSCs with EGM and MMP-2 siRNA ( B ), and AMSCs with EGM and MMP-14 siRNA ( C ). Flow cytometry data were analyzed to show the significant differences with or without U0126 ( D ). * p < 0.05, ** p < 0.01, *** p < 0.001. EBM endothelial cell basal medium, EGM endothelial cell growth medium, MMP matrix metalloproteinase, ERK extracellular signal-regulated kinase, HUVEC human umbilical vein endothelial cell
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
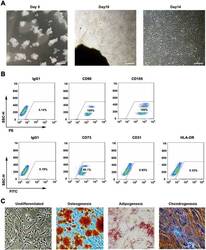
- Fig. 1 WJMSCs isolation and characterization. a Primary cell isolation procedure from Wharton''s jelly tissue. The migrated cells exhibited typical fibroblast-like morphology. Scale bar, 500 mum. b Flow cytometry analysis of P4 cells using mesenchymal stem cell markers (CD90, CD105, CD73), endothelial cell marker (CD31), and MHC class II protein HLA-DR. Isotypic antibodies (IgG1-PE and IgG1-FITC) were used as negative controls. c Representative stained images show that the fourth passage WJMSCs could differentiate into osteocytes (Alizarin Red S), adipocytes (Oil Red O), and chondrocytes (Alcian blue). Scale bar, 100 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
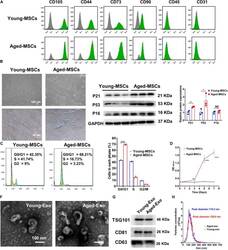
- FIGURE 1 Characterization of young and aged MSCs and exosomes. (A) Surface marker profiling of young-MSCs and aged-MSCs. (B) SA-beta-Gal staining showed that senescence increased significantly in aged MSCs. (C) Representative immunoblot images and quantitative analysis of p21, p53, and p16 protein level in young and aged-MSCs. ( n = 3). (D) Quantitation of cell cycle phases by propidium iodide staining. ( n = 3). (E) The CCK-8 assay showed that aged MSCs grew more slowly than young MSCs. ( n = 6). (F) Young and aged exosomes were observed using TEM. (G) The exosome surface markers were analyzed by Western blot. (H) Nanoparticle tracking analysis was used to analyze the particle size and concentration of Young-Exo and Aged-Exo. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; NS, not significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5 siRNA transfection and immunophenotyping of differentiated adipose-derived mesenchymal stem cells (AMSCs); (I) : MMP-2 (A) and MMP-14 (B) silencing by siRNA transfection with EGM compared to AMSCs with EGM and EGM plus scrambled siRNA (negative control). GAPDH was used as a housekeeping gene (*, p < .05; **, p < .01; ***, p < .001). (II) : Flow cytometric analysis of PECAM1 (CD31) in five different groups; control group was the undifferentiated cells with EBM (A), AMSCs with differentiation medium EGM (B), AMSCs with differentiation medium EGM and MMP-2 siRNA (C), AMSCs with differentiation medium EGM and MMP-14 siRNA (D), and HUVECs as the positive control (E). Flow cytometry data were analyzed to show the significant differences between the groups (F). (III) : Flow cytometric analysis of VE-Cadherin (CD144) in five different groups; control group was the undifferentiated cells with EBM (A), AMSCs with differentiation medium EGM (B), AMSCs with differentiation medium EGM and MMP-2 siRNA (C), AMSCs with differentiation medium EGM and MMP-14 siRNA (D), and HUVECs as the positive control (E). Flow cytometry data were analyzed to show the significant differences between the groups (F). (*, p < .05; **, p < .01; ***, p < .001). Abbreviations: CD, cluster of differentiation; EBM, endothelial basal medium; EGM, endothelial growth medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HUVECs, human umbilical vein endothelial cells; MMP, matrix metalloproteinases.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
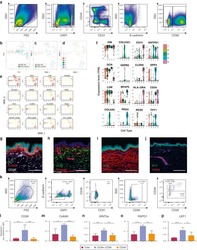
- Figure 7 Immunostaining of VEGFR2 and immunophenotyping of differentiated adipose-derived mesenchymal stem cells (AMSCs) after VEGFR2 kinase inhibition; (I) : Immunofluorescence staining for VEGFR2. AMSCs in EGM showed significant increases in the expression of VEGFR2 (B & E) compared to endothelial basal medium group (A & E). AMSCs cultured with EGM and MMP-2 siRNA showed significantly higher fluorescence intensity of VEGFR2 in comparison to the EGM cultured cells (C & E). AMSCs cultured with EGM and MMP-14 siRNA showed the greatest positive staining of VEGFR2 compared to that of EGM and EGM plus MMP2 siRNA (D & E). Fluorescence intensity was measured to show the significant differences between the groups using ImageJ software (E). (II) : Flow cytometric analysis of PECAM1 (CD31) in three different groups; control group was the differentiated cells with EGM and 5 muM of VEGFR2 inhibitor (A), AMSCs with differentiation medium EGM, MMP-2 siRNA and 5 muM of VEGFR2 inhibitor (B) and AMSCs with EGM, MMP-14 siRNA and 5 muM of VEGFR2 inhibitor (C). Flow cytometry data were analyzed to show the significant differences between the groups in comparison to the same groups without VEGFR2 inhibitor (D). (III) : Flow cytometric analysis of VE-Cadherin (CD144) in three different groups; the differentiated cells with EGM and 5 muM of VEGFR2 inhibitor (A), AMSCs with differentiation medium EGM, MMP-2 siRNA and 5 muM of VEGFR2 inhibitor (B) and AMSCs with EGM, MMP-14 siRNA and 5 muM of VEGFR2
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 Changes in the expression rate of CD31 in the peripheral blood of rats. Rats were randomly divided into the control, sham surgery and S2h, S8h, S24h, S48h and S72h groups, which were examined 2, 8, 24, 48 and 72 h after deep vein thrombosis modeling surgery, respectively. Flow cytometry was used to analyze the positive rate of the peripheral blood endothelial cell marker CD31. (A) Representative flow cytometry plots for each group. (B) Statistical analysis of the CD31 positive rate in each group. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
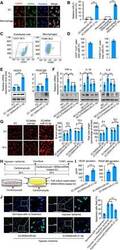
- Fig. 1 In vitro bioactivity of the MSN/miR-21-5p complex. ( A ) In vitro uptake of the MSN/miR-21-5p complex by adherent endothelial cells (ECs) and macrophages (MCs). ( B ) In vitro transfection efficiency of miR-21-5p was determined by quantifying the miRNA level using real-time quantitative PCR. ( C ) Representative flow cytometry analysis of CD31 levels in ECs and F4/80 levels in MCs. ( D ) In vitro uptake of the MSN/miR-21-5p complex by ECs and MCs was determined by quantifying the double-positive cells (CD31 or F4/80 and Cy3) using flow cytometric analysis. The protein expression levels of VEGFA and PDGF-BB in endothelial cells ( E ) and tumor necrosis factor-alpha (TNF-alpha), interleukin-1beta (IL-1beta), and IL-6 in macrophages ( F ) were determined by the real-time quantitative PCR and Western blot analysis. ( G ) The endothelial cells that formed three-dimensional (3D) capillary-like tubular structures were evaluated at indicated times (8 and 16 hours). ( H ) Schematic diagram of the experimental setup. TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling. ( I ) Apoptosis-positive cardiomyocytes from these treatment groups were further quantified. ( J ) Protein levels of secreted proangiogenic factors were determined by enzyme-linked immunosorbent assay (ELISA) analysis of cell supernatants from the MSN/miRNA-treated ECs (scale bars, 50 mum). * P < 0.05 and *** P < 0.01. All experiments were carried out in triplicate. n
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 1 Primary human adipose-derived cells cultured in hypoxia (hAMSCs-H) and normoxia (hAMSCs-N) are both MSCs but normoxia-cultured cells show increased signs of senescence, such as increased area and elongated morphology, compared with hypoxia-cultured cells. ( a ) hAMSCs were isolated from human fat tissue and cultured in hypoxic (1.5% oxygen) or normoxic (21% oxygen) conditions in vitro . The viability, mobility, tumor tropism, safety, and tumorigenic potential were subsequently compared in vitro and in vivo . ( b ) Differentiation assay. hAMSCs were cultured in control media and differentiation media for 3 weeks, 10 days after the second passage. Three different stains were performed to assess differentiation capabilities (scale bar, 100 mu m). ( c ) Flow cytometric analysis was performed to confirm the absence of CD31-, CD34-, and CD45-positive cells in both cell cultures. In addition, primary hAMSC cultures expressed high levels of CD73, CD90, and CD105, both in hypoxic and normoxic culture conditions at day 10 after passage 2. ( d ) Representative images of cell morphologies of hAMSCs on 2D surface (scale bar, 200 mu m). ( e ) Schematic of 3D-nanopatterned surface used to assess morphology and motility. ( f ) Images of cell morphologies of hAMSCs on 3D-nanopatterned surface (scale bar, 200 mu m). ( g - j ) The length, width, area, and length-to-width ratio were measured and compared after cell aligned on the nanopattern surface. Error bars represent S.E.M. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 Hypoxia-cultured primary human adipose-derived mesenchymal stem cells (hAMSCs-H) retain a greater proliferation capacity compared with normoxia-cultured primary hAMSCs (hAMSCs-N) when exposed to GBM media. hAMSCs-H maintain stem cell characteristics when exposed to GBM media. ( a ) Representative MRI of GBM from a patient. ( b ) Schema showing the collection of GBM CM and culture of hAMSCs in filtered GBM CM for proliferation and migration assays. ( c ) MTT assay was used to determine the effects of hypoxic conditions on the proliferative capacity of primary hAMSCs in GBM CM. In GBM CM, hAMSCs-H showed greater proliferation at day 10 and 15 compared with hAMSCs-N. ( d ) Ki-67 immunostaining was performed to quantify the number of proliferating cells in GBM CM. Proliferative capacities of hAMSCs-H and hAMSCs-N are shown in GBM CM (normalized to hAMSC-N proliferative capacity in control media). In GBM CM, hAMSCs-H had a greater proportion of proliferating cells than hAMSCs-N. ( e) Differentiation assay. hAMSCs were cultured in control media, differentiation media, and GBM CM for 3 weeks, 10 days after the second passage. Three stainings were performed to assess the differentiation capabilities (scale bar, 100 mu m). Both hAMSCs-N and hAMSCs-H maintained tri-lineage differentiation capability in GBM CM. ( f ) Flow cytometric analysis for CD31, CD34, CD45, CD73, CD90, and CD105 in hAMSC-N and hAMSC-H cultures after exposure to GBM CM for 20 days. hAMSCs-H maintained MSC
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
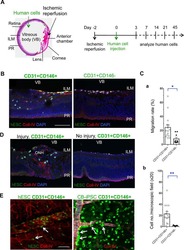
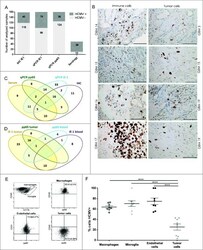
- Figure 1. HCMV positivity in GBM determined comparatively by serology, qPCR and IHC. GBM patients were investigated for presence of HCMV pp65 and IE-1 expression by (A) qPCR, IHC and serology (dark gray bars = positive samples and light gray bar = negative samples) and (B) IE-1 expression on macrophage/myeloid derived cells (left panel) and on tumor cells (right panel). Magnification 400X, Scale bar 100 mum. (C) Venn diagram showing comparative HCMV serology (IgG), qPCR (pp65), qPCR (IE-1) and IHC (IE-1) detection in GBM tissue and blood. (D) Venn diagram showing comparative HCMV pp65 and IE-1 qPCR in GBM patients' blood and tumor. (E) Representative dotplots showing (left to right) macrophages (CD45 bright CD11b bright ) and microglia (CD45 dim CD11b bright ) within tumor biopsies cells; pp65 vs. CD45 within macrophages of tumor biopsies; pp65 vs . CD31 within CD45 - tumor biopsy cells; and pp65 vs . CD45 within CD45 - tumor biopsy cells. (F) % mean +- SEM of pp65 + cells within CD45 bright CD11b bright macrophages, CD45 dim CD11b bright microglia, CD45-CD31+ endothelial cells and CD45- tumor cells. (2-way ANOVA, Bonferroni's multiple comparison, **** P < 0.0001). IHC = immunonohistochemistry; IE-1 = immediate early -1.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
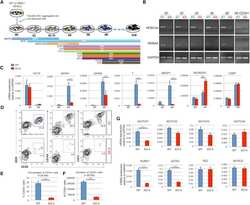
- Figure 3 HEB -/- hESCs Display Defects in Mesoendodermal Induction and Early Hematopoietic Differentiation (A) Experimental overview of embryoid body (EB) formation and differentiation. BMP4, bone morphogenetic protein 4; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; IL, interleukin; EPO, erythropoietin; SCF, stem cell factor; IGF1, insulin-like growth factor 1; FLT3L, FMS-like tyrosine kinase 3 ligand; TPO, thrombopoietin. (B) Reverse-transcriptase PCR analysis of HEB transcript (HEBCan, canonical; HEBAlt, alternative) expression at various stages of EB differentiation, and in sorted day-8 (d8) CD34 + cells (last column). GAPDH was measured as a loading control. (C) qRT-PCR analysis for the expression of pluripotency and differentiation markers in undifferentiated hESCs (day 0 [d0]) versus d4 EB-derived cells. (D) Flow-cytometric analysis of CD34 and KDR, CD144, and CD31 expression on d8 EB-derived cells. (E and F) Percentages (E) and numbers (F) of CD34 + cells in d8 EBs. (G) qRT-PCR analysis of the expression of mesodermal and hematopoietic genes in CD34 + cells. For qRT-PCR graphs, mRNA levels are shown relative to GAPDH. Error bars represent mean +- SD (n = 3 independent experiments). ** p < 0.01; *** p < 0.005 by Student's t test. Images in (B) and plots in (D) are representative of three independent experiments. See also Figure S4 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
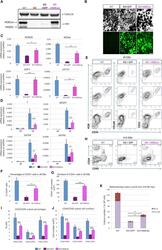
- Figure 6 Ectopic Expression of HEBCan in HEB -/- hESCs Restores Lineage-Specific Gene Expression and Hematopoietic Specification (A) Western blot analysis for HEB expression in WT, KO (HEB -/- ), KO + GFP (HEB -/- hESCs transduced with GFP control vector) and KO + HEBCan (HEB -/- hESCs transduced with HEBCan-encoding vector) hESCs. (B) Bright-field (top) and fluorescent (bottom) images of day-8 (d8) EBs derived from HEB -/- hESCs transduced with control or HEBCan-expressing lentiviral particles. Scale bar, 100 mum. (C and D) qRT-PCR analysis for the expression of pluripotency-associated genes (C) and mesoendodermal genes (D) in WT, KO + GFP, and KO + HEBCan hESC-derived cells at d0 and d4 of EB culture. mRNA levels are shown relative to GAPDH. (E) Flow-cytometric analysis of CD34 and KDR, CD144, and CD31 on WT, KO + GFP, and KO + HEBCan d8 EB-derived cells. (F and G) Percentages (F) and numbers (G) of CD34 + cells in WT, KO + GFP, and KO + HEBCan d8 EBs. (H) Flow-cytometric analysis for CD34 and CD45 on WT, KO + GFP, and KO + HEBCan d18 EB-derived cells. (I and J) Percentages (I) and numbers (J) of CD34/CD45 subsets in WT, KO + GFP, and KO + HEBCan d18 EB-derived cells. (K) Numbers of erythroid (BFU-E) and myeloid (CFU-GM) arising from unfractionated WT, KO + GFP, and KO + HEBCan d18 EBs. Error bars represent mean +- SD (n = 3 independent experiments). * p < 0.05, ** p < 0.01, *** p < 0.005 by Student's t test. Images in (A) and (B) and plots in (E) and (H) are represent
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
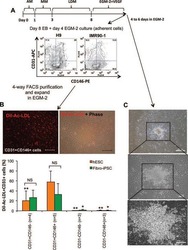
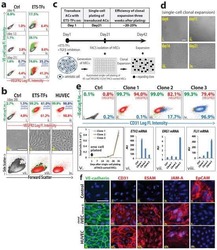
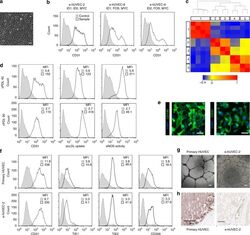
- Fig. 3 Comparable phenotype of primary and expanded endothelial cells. a Phase contrast microscopy shows the expandable HUVEC cell line e-hUVEC-2 (MYC, ID1, and ID2). Scale bar 100 um. b CD31 expression of human endothelial cell populations immortalized with three different gene sets as indicated. c Global gene expression analysis was performed on three different HUVEC lines (e-hUVEC-2, 8 and 9; in duplicate) (3, 4, 5, respectively) and two independent primary HUVEC populations (2) as well as four primary gingiva fibroblast populations (1). Expression data was processed with GeneSpring 11.5.1 software and a Standard Pearson Correlation was determined for each gene versus all other genes. The correlation heatmap depicts the pair-wise correlation coefficient between the given samples and displays the relationship between the different samples. The samples are clustered based on the pair-wise correlation coefficients between all entities. d Phenotypic stability of e-hUVEC-2 cells after 45 and 90 cumulative population doublings was evaluated by CD31 (also known as PECAM1) expression, acetylated LDL uptake, and eNOS activity. Gray fill: antibody isotype control; black outline: stained sample. MFI: median fluorescence intensity. e Immunofluorescence-based detection of CD31 and CD146 (also known as MCAM) in expandable HUVECs counterstained for nuclei with DAPI. Scale bars, 100 um. f The phenotype and the functionality of cell line e-hUVEC-2 (cumulative population doubling 80) was co
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry