Antibody data
- Antibody Data
- Antigen structure
- References [10]
- Comments [0]
- Validations
- Western blot [5]
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Other assay [8]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-21349 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Complement C3 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Description
- Recommended positive controls: Huh7, HepG2. Store product as a concentrated solution. Centrifuge briefly prior to opening the vial.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 µL
- Concentration
- 0.08 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Activated endothelial cells induce a distinct type of astrocytic reactivity.
Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: microglia-astrocyte involvement in remyelination.
The Retropepsin-Type Protease APRc as a Novel Ig-Binding Protein and Moonlighting Immune Evasion Factor of Rickettsia.
Bacillus anthracis Poly-γ-D-Glutamate Capsule Inhibits Opsonic Phagocytosis by Impeding Complement Activation.
Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like BPH/5 Mouse Prior to and During Pregnancy.
IgE Contributes to Atherosclerosis and Obesity by Affecting Macrophage Polarization, Macrophage Protein Network, and Foam Cell Formation.
Loss of Complement Factor H impairs antioxidant capacity and energy metabolism of human RPE cells.
A1 reactive astrocytes and a loss of TREM2 are associated with an early stage of pathology in a mouse model of cerebral amyloid angiopathy.
Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice.
Immunity against the Obligate Intracellular Bacterial Pathogen Rickettsia australis Requires a Functional Complement System.
Taylor X, Cisternas P, Jury N, Martinez P, Huang X, You Y, Redding-Ochoa J, Vidal R, Zhang J, Troncoso J, Lasagna-Reeves CA
Communications biology 2022 Mar 29;5(1):282
Communications biology 2022 Mar 29;5(1):282
Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: microglia-astrocyte involvement in remyelination.
Zheng J, Lu J, Mei S, Wu H, Sun Z, Fang Y, Xu S, Wang X, Shi L, Xu W, Chen S, Yu J, Liang F, Zhang J
Journal of neuroinflammation 2021 Feb 15;18(1):43
Journal of neuroinflammation 2021 Feb 15;18(1):43
The Retropepsin-Type Protease APRc as a Novel Ig-Binding Protein and Moonlighting Immune Evasion Factor of Rickettsia.
Curto P, Barro A, Almeida C, Vieira-Pires RS, Simões I
mBio 2021 Dec 21;12(6):e0305921
mBio 2021 Dec 21;12(6):e0305921
Bacillus anthracis Poly-γ-D-Glutamate Capsule Inhibits Opsonic Phagocytosis by Impeding Complement Activation.
Sharma S, Bhatnagar R, Gaur D
Frontiers in immunology 2020;11:462
Frontiers in immunology 2020;11:462
Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like BPH/5 Mouse Prior to and During Pregnancy.
Olson KN, Reijnders D, Gomes VCL, Hebert RC, Liu CC, Stephens JM, Redman LM, Douglas NC, Sones JL
Biology 2020 Sep 22;9(9)
Biology 2020 Sep 22;9(9)
IgE Contributes to Atherosclerosis and Obesity by Affecting Macrophage Polarization, Macrophage Protein Network, and Foam Cell Formation.
Zhang X, Li J, Luo S, Wang M, Huang Q, Deng Z, de Febbo C, Daoui A, Liew PX, Sukhova GK, Metso J, Jauhiainen M, Shi GP, Guo J
Arteriosclerosis, thrombosis, and vascular biology 2020 Mar;40(3):597-610
Arteriosclerosis, thrombosis, and vascular biology 2020 Mar;40(3):597-610
Loss of Complement Factor H impairs antioxidant capacity and energy metabolism of human RPE cells.
Armento A, Honisch S, Panagiotakopoulou V, Sonntag I, Jacob A, Bolz S, Kilger E, Deleidi M, Clark S, Ueffing M
Scientific reports 2020 Jun 25;10(1):10320
Scientific reports 2020 Jun 25;10(1):10320
A1 reactive astrocytes and a loss of TREM2 are associated with an early stage of pathology in a mouse model of cerebral amyloid angiopathy.
Taylor X, Cisternas P, You Y, You Y, Xiang S, Marambio Y, Zhang J, Vidal R, Lasagna-Reeves CA
Journal of neuroinflammation 2020 Jul 25;17(1):223
Journal of neuroinflammation 2020 Jul 25;17(1):223
Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice.
Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, Di Z, Liu Z, Baskys A, Liu W, Wu S, Long Q
Theranostics 2019;9(20):5956-5975
Theranostics 2019;9(20):5956-5975
Immunity against the Obligate Intracellular Bacterial Pathogen Rickettsia australis Requires a Functional Complement System.
Riley SP, Fish AI, Del Piero F, Martinez JJ
Infection and immunity 2018 Jun;86(6)
Infection and immunity 2018 Jun;86(6)
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of C3 using Various whole cell extracts (30 µg). Samples were loaded onto a 5% SDS-PAGE gel and probed with a C3 polyclonal antibody (Product # PA5-21349) at a dilution of 1:1000.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of C3 using A) 30 µg 293T whole cell extract and B) 30 µg whole cell extract of human C3-transfected 293T cells. Samples were loaded onto a 5% SDS-PAGE gel and probed with a C3 polyclonal antibody (Product # PA5-21349) at a dilution of 1:20000.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
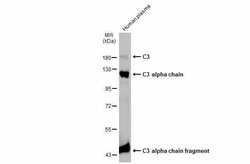
- Experimental details
- Western Blot analysis of Complement C3 was performed by separating 30 µg of Human plasma by 7.5% SDS-PAGE. Proteins were transferred to a membrane and probed with a Complement C3 Polyclonal Antibody (Product # PA5-21349) at a dilution of 1:10000. The HRP-conjugated anti-rabbit IgG antibody was used to detect the primary antibody.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western Blot analysis of Complement C3 was performed by separating 30 µg of various whole cell extracts by 5% SDS-PAGE. Proteins were transferred to a membrane and probed with a Complement C3 Polyclonal Antibody (Product # PA5-21349) at a dilution of 1:20,000.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot was performed using Anti-Complement C3 Polyclonal Antibody (Product # PA5-21349) and a 187 kDa band corresponding to Complement C3 was observed across cell lines tested. The expression of Complement C3 was upregulated with LPS and IL-1beta treatment. Whole cell extracts (30 µg lysate) of Hep G2 (Lane 1), Hep G2 treated with Protein transport inhibitor (1X for 4hr) (Lane 2), Hep G2 treated with LPS (10 ng/mL for 18hr) and Protein transport inhibitor (1X for 4 hr) (Lane 3), A549 (Lane 4) and A549 treated with IL-1beta (5 ng/mL 48hr) (Lane 5) were electrophoresed using NuPAGE™ 3-8% Tris-Acetate Protein Gel (Product # EA0378BOX). Resolved proteins were then transferred onto a Nitrocellulose membrane (Product # IB23001) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (1:3000 dilution) and detected by chemiluminescence with Goat anti-Rabbit IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A27036, 1:4000 dilution) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunocytochemistry-Immunofluorescence analysis of Complement C3 was performed in HeLa cells fixed in 4% paraformaldehyde at RT for 15 min. Green: Complement C3 Polyclonal Antibody (Product # PA5 21349) diluted at 1:200. Blue: Hoechst 33342 staining.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescence analysis of Complement C3 was performed using 70% confluent log phase Hep G2 cells treated with 10 ng/mL of LPS for 18 hours. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Complement C3 Polyclonal Antibody (Product # PA5-21349) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e represents untreated cells with weak cytoplasmic signal. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- C3 antibody [C3], C-term detects C3 protein at cytoplasm in mouse brain by immunohistochemical analysis. Sample: Paraffin-embedded mouse brain. C3 antibody [C3], C-term (Product # PA5-21349) diluted at 1:500. Antigen Retrieval: Citrate buffer, pH 6.0, 15 min.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 3 A1 Astrocytes are highly abundant in Tg-FDD. a Double-stained immunofluorescent images of complement component 3 (C3, red) and astrocytes (GFAP, green) in the hippocampus of WT and Tg-FDD mice. C3 and GFAP immunoreactivity overlay (Merge). b Quantification of C3 + GFAP + cell % from 3 regions per animal of the hippocampus of 4 WT and 3 Tg-FDD mice. c Quantification of % colocalization area from the hippocampus of 4 WT and 3 Tg-FDD mice. d Double-stained immunofluorescent images of complement component 3 (C3, red) and astrocytes (GFAP, green) in the cerebellum of WT and Tg-FDD mice. C3 and GFAP immunoreactivity overlay (Merge). e Quantification of C3 + GFAP + cells % from 3 regions per animal of the cerebellum of 4 WT and 3 Tg-FDD mice. f Quantification of % colocalization area from the cerebellum of 4 WT and 3 Tg-FDD mice. a and d are representative images of the brain regions of 9-month-old mice. Colocalization analysis (CC) was performed to determine pixel intensity correlation between C3 and GFAP signals. White pixels indicate colocalization between C3 and GFAP signal. Plot profiles of representative C3 and GFAP intensities show higher overlapping of C3 with GFAP in Tg-FDD mice in comparison with WT mice, indicating an increase of C3 positive astrocytes. Results are shown as the mean +- SEM of n = 3-4. Asterisks indicate significant differences, where **** p < 0.0001 by unpaired Student's t test. Scale bar 100 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
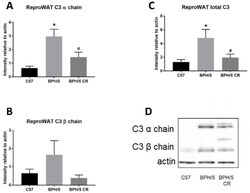
- Experimental details
- Figure 2 Complement factor 3 (C3) protein is increased in BPH/5 reproductive (repro) WAT in early pregnancy. ( A ) Quantification of C3 alpha chain, ( B ) C3 beta chain, and ( C ) combined (alpha chain and beta chain) C3 levels were measured in reproductive WAT of ad libitum-fed C57, ad libitum-fed BPH/5, and calorie-restricted (CR) BPH/5 mice at e7.5 ( n = 3, * p < 0.05 vs. C57, # p < 0.05 vs. BPH/5). Data are expressed as mean +- SEM. ( D ) Representative Western blot gel of actin and C3 denatured protein levels.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 7 PEG-CeNP treatment protected against A1 astrocyte alteration after ICH. Representative RT-qPCR analysis of A1 astrocyte markers expression. a C3 mRNA expression. b Amigo2 mRNA expression. c Serping1 mRNA expression. n = 6/group, * p < 0.01 versus Sham. ^ p < 0.01 versus ICH 3d. # p < 0.01 versus ICH 7d. d C3 mRNA expression in PEG-CeNP-treated mice at 7 days post ICH. n = 6/group, * p < 0.01 versus Sham, # p < 0.01 versus ICH + vehicle. e Representative images of GFAP and C3 double immunostaining in cultured astrocytes (treated with H 2 O 2 ). Lens: x 400; Scale bar: 50 mum. f Representative images of GFAP and C3 double immunostaining in brain sections at 7 days post ICH. Lens: x 100, x 200; Scale bar: 200 mum (yellow), 100 mum (white). g C3 mRNA expression in cultured astrocytes. n = 6/group, * p < 0.05 versus Control, # p < 0.05 versus H 2 O 2 + vehicle. h Representative Western blot band showed the expression of nuclear NF-kappaB p65 in cultured astrocytes, n = 6/group, * p < 0.05 versus Control, # p < 0.05 versus H 2 O 2 + vehicle. i Representative images of NF-kappaB p65 and GFAP double immunostaining in cultured astrocytes (treated with H 2 O 2 ). Lens: x 400; Scale bar: 25 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
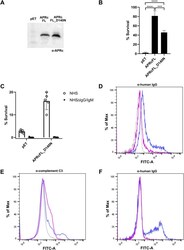
- Experimental details
- FIG 9 APRc protects E. coli from complement-mediated killing, and interaction with IgG contributes to serum resistance. (A) Western blot analysis with anti-APRc antibody of total protein extracts from BL21 Star(DE3) E. coli strain expressing the empty vector backbone pET28a (pET) or the plasmid encoding untagged full-length APRc wild type (APRcFL) and the corresponding catalytic mutant (APRcFL_D140N). (B) The serum-sensitive BL21 Star(DE3) E. coli strain expressing the empty vector (pET) or the plasmid encoding APRcFL and APRcFL_D140N was independently incubated 1:1 with PBS or in PBS containing 40% NHS for 1 h. After incubation, the samples were serially diluted, plated onto LB agar plates, and incubated overnight at 37degC. The average number of CFU per milliliter was calculated from the replicate plate counts. The data are presented as survival rate, which was calculated as the percentage of the original cell number at T 0 (considered 100% survival). Data represent the mean +- SD from 4 independent biological replicates. Significance was determined using a one-way ANOVA followed by Tukey multiple-comparison test using GraphPad Prism 8 (***, P < 0.001; ****, P < 0.0001). (C) BL21 Star(DE3) E. coli strain expressing the empty vector (pET) or the plasmid encoding APRcFL_D140N was independently incubated 1:1 with PBS or in PBS containing 40% NHS or 40% NHSDeltaIgG/IgM for 1 h. After incubation, samples were treated as described for panel B. Data represent the mean +- SD from 4
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
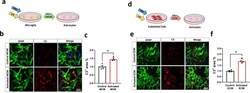
- Experimental details
- Fig. 1 C3+ Astrocytes are induced by LPS-Activated ECM. a Schematic diagram showing treatment of astrocytes with control or activated microglia conditioned medium (MCM) b Double immunofluorescence of complement component 3 (C3, red) and astrocytes (GFAP, green) treated with control or activated MCM in primary astrocyte cultures. C3 and GFAP immunoreactivity overlay (merge) c Quantification of C3 + area (%) of control or activated MCM treated astrocytes. d Schematic diagram showing treatment of astrocytes with control or activated endothelial conditioned medium (ECM) e Double immunofluorescence of complement component 3 (C3, red) and astrocytes (GFAP, green) treated with control or activated ECM in primary astrocyte cultures. C3 and GFAP immunoreactivity overlay (merge) f Quantification of C3 + area (%) of control or activated ECM treated astrocytes. The number of cells analyzed was 20 per image from 10 different images per culture from 4 independent cultures. Results are shown as the median +- IQR of n = 4. Asterisks indicate significant differences, where * p < 0.05 by Mann-Whitney test. Scale bar 50 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Reactive endothelial cells induce C3+ astrocytes distinct from reactive microglia. a , c Triple immunofluorescence of astrocytes (GFAP, cyan), complement component 3 (C3, green) and GBP2 (red) in primary astrocyte cultures. b , d Quantification of GBP2 + area (%) of control or activated MCM/ECM-treated astrocytes, an increased presence of GBP2 immunoreactivity is observed only in astrocytes stimulated with activated MCM. e , g Triple immunofluorescence of astrocytes (GFAP, cyan), complement component 3 (C3, green) and Decorin (red) in primary astrocyte cultures. f , h Quantification of Decorin + area (%) of control or activated MCM/ECM-treated astrocytes, an increased presence of Decorin immunoreactivity is observed exclusively in astrocytes stimulated with activated ECM. The number of cells analyzed was 20 per image from 10 different images per culture from 4 independent cultures. Results are shown as the median +- IQR of n = 4. Asterisks indicate significant differences were, * P < 0.05 by Mann-Whitney test. Scale bar 50 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Decorin+ astrocytes are associated with vascular amyloid in vivo. a Triple immunofluorescence of amyloid (Thio-S, green), astrocytes (GFAP, cyan), and Decorin (red) in the cortex of 15-month-old Tg-FDD, APP/PS1 mice and WT controls. Major presence of Decorin immunoreactivity is observed in the perivascular region of Thio-S positive vasculature in Tg-FDD mice. Yellow arrows indicate Decorin + , GFAP + cells and purple arrows indicate Decorin-, GFAP + cells. Colocalization analysis (CC) was performed to determine pixel intensity correlation between GFAP and Decorin. White pixels indicate colocalization between GFAP and Decorin signal and white dashed arrows represent distance in plot profiles showing overlapping intensities of astrocytes (GFAP, cyan) and Decorin (red). b Quantification of the proportion of Decorin + cells in Tg-FDD, APP/PS1 and WT mice. c Triple immunofluorescence of astrocytes (GFAP, green), complement component 3 (C3, cyan), and Decorin (red) in the cortex of 15-month-old Tg-FDD and APP/PS1 mice. Yellow arrows indicate C3 + , Decorin + , GFAP + cells and purple arrows indicate C3 + , Decorin-, GFAP + cells. d Quantification of the proportion of C3 + Decorin + astrocytes versus C3 + astrocytes in Tg-FDD, APP/PS1 and WT mice. For quantifications, eight to ten images were used for each experiment ( n = 3), and 200 cells were counted. All are representative images of 15-month-old Tg-FDD, APP/PS1 or WT mice. Scale bar 25 or 50 mum, respectively.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 MSCs-Exo protect against LPS-induced astrocytic activation and inflammatory responses in vitro . Astrocytic activation and inflammatory responses were examined using immunohistochemistry, Western blotting, or ELISA. (A-L) Immunostaining images of GFAP (A-H, red, a marker of reactive astrocytes), C3 (A-D, green, a marker A1 astrocytes), CD81 (E-H, green, a regulator of astrogliosis), and ki67 (I-L, green, a marker of cell proliferation). (M-P) Statistical analysis of the fluorescence intensity of GFAP (M), C3 (N), CD81 (O), and ki67 (P) in the Control, Control+MSC-Exo, LPS, and LPS+MSC-Exo group. (Q) Western blots of the relative expression of GFAP (R), C3 (S), and CD81 (T) in each group. (U-W) Concentration of inflammatory cytokines secreted by astrocytes including TNF-alpha (U), IL-1beta (V), and IL-6 (W) determined by ELISA. Histograms of statistical difference between groups are shown. Scale bars: A-H, 50 mum; I-L, A1-H1 and A2-H2, 20 mum.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation