Antibody data
- Antibody Data
- Antigen structure
- References [33]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [1]
- Other assay [14]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 35-8200 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- COX2 Monoclonal Antibody (COX 229)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- COX 229
- Vial size
- 100 µg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references 20-HETE Participates in Intracerebral Hemorrhage-Induced Acute Injury by Promoting Cell Ferroptosis.
Interleukin-26 Has Synergistic Catabolic Effects with Palmitate in Human Articular Chondrocytes via the TLR4-ERK1/2-c-Jun Signaling Pathway.
Secreted Frizzled-Related Protein 1 as a Biomarker against Incomplete Age-Related Lobular Involution and Microcalcifications' Development.
Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer.
N-AS-triggered SPMs are direct regulators of microglia in a model of Alzheimer's disease.
Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes.
Lactobacillus casei Strain Shirota Enhances the In Vitro Antiproliferative Effect of Geniposide in Human Oral Squamous Carcinoma HSC-3 Cells.
COX-2 gene expression and methylation profile in Sapajus apella as an experimental model for gastric adenocarcinoma.
Association between local inflammation and breast tissue age-related lobular involution among premenopausal and postmenopausal breast cancer patients.
Evaluation of Antioxidant Intakes in Relation to Inflammatory Markers Expression Within the Normal Breast Tissue of Breast Cancer Patients.
Increased COX-2 expression in epithelial and stromal cells of high mammographic density tissues and in a xenograft model of mammographic density.
COX-2 overexpression and -8473 T/C polymorphism in 3' UTR in non-small cell lung cancer.
Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson's disease.
Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-κB and HIF-1α signaling pathways.
A quantitative proteomic analysis uncovers the relevance of CUL3 in bladder cancer aggressiveness.
GRSF1 regulates RNA processing in mitochondrial RNA granules.
Roles of E-cadherin and cyclooxygenase enzymes in predicting different survival patterns of optimally cytoreduced serous ovarian cancer patients.
Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure.
Cyclooxygenase-2 up-regulates vascular endothelial growth factor via a protein kinase C pathway in non-small cell lung cancer.
Enhancement of osteoclastogenic activity in osteolytic prostate cancer cells by physical contact with osteoblasts.
Dissecting spatio-temporal protein networks driving human heart development and related disorders.
Expression of cyclooxygenase-2 in choroidal neovascular membranes from age-related macular degeneration patients.
Prognostic significance of cyclooxygenase-2 overexpression in glottic cancer.
The use of a cyclooxygenase-2 inhibitor (Nepafenac) in an ocular and metastatic animal model of uveal melanoma.
Expression of cyclo-oxygenase-2 in macrophages associated with cutaneous melanoma at different stages of progression.
Cyclooxygenase-2 (COX-2) overexpression in meningiomas: real time PCR and immunohistochemistry.
Expression of protease-activated receptor-1 and -2 in orofacial granulomatosis.
The expression of cyclooxygenase 2 in retinoblastoma: primary enucleated eyes and enucleation after conservative treatment.
Pulmonary cryptosporidiosis: role of COX2 and NF-kB.
Cyclooxygenase-2 (COX-2) overexpression in childhood brain tumors.
Cyclooxygenase-2 and inducible nitric oxide synthase expression in thyroid neoplasms and their clinicopathological correlation.
Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid.
Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo.
Han R, Wan J, Han X, Ren H, Falck JR, Munnuri S, Yang ZJ, Koehler RC
Frontiers in neurology 2021;12:763419
Frontiers in neurology 2021;12:763419
Interleukin-26 Has Synergistic Catabolic Effects with Palmitate in Human Articular Chondrocytes via the TLR4-ERK1/2-c-Jun Signaling Pathway.
Chen YT, Wang CC, Cheng CP, Liu FC, Lee CH, Lee HS, Peng YJ
Cells 2021 Sep 21;10(9)
Cells 2021 Sep 21;10(9)
Secreted Frizzled-Related Protein 1 as a Biomarker against Incomplete Age-Related Lobular Involution and Microcalcifications' Development.
Clemenceau A, Hanna M, Ennour-Idrissi K, Burguin A, Diorio C, Durocher F
Cancers 2020 Sep 21;12(9)
Cancers 2020 Sep 21;12(9)
Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer.
Lee J, You JH, Kim MS, Roh JL
Redox biology 2020 Oct;37:101697
Redox biology 2020 Oct;37:101697
N-AS-triggered SPMs are direct regulators of microglia in a model of Alzheimer's disease.
Lee JY, Han SH, Park MH, Song IS, Choi MK, Yu E, Park CM, Kim HJ, Kim SH, Schuchman EH, Jin HK, Bae JS
Nature communications 2020 May 12;11(1):2358
Nature communications 2020 May 12;11(1):2358
Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes.
Albouchi F, Avola R, Dico GML, Calabrese V, Graziano ACE, Abderrabba M, Cardile V
Molecules (Basel, Switzerland) 2018 Oct 2;23(10)
Molecules (Basel, Switzerland) 2018 Oct 2;23(10)
Lactobacillus casei Strain Shirota Enhances the In Vitro Antiproliferative Effect of Geniposide in Human Oral Squamous Carcinoma HSC-3 Cells.
Qian Y, Song JL, Sun P, Yi R, Liu H, Feng X, Park KY, Zhao X
Molecules (Basel, Switzerland) 2018 May 3;23(5)
Molecules (Basel, Switzerland) 2018 May 3;23(5)
COX-2 gene expression and methylation profile in Sapajus apella as an experimental model for gastric adenocarcinoma.
Rosário Pinheiro DD, Harada ML, Rodriguez Burbano RM, Nascimento Borges BD
Genetics and molecular biology 2018 Apr. Jun;41(2):496-501
Genetics and molecular biology 2018 Apr. Jun;41(2):496-501
Association between local inflammation and breast tissue age-related lobular involution among premenopausal and postmenopausal breast cancer patients.
Hanna M, Dumas I, Orain M, Jacob S, Têtu B, Sanschagrin F, Bureau A, Poirier B, Diorio C
PloS one 2017;12(8):e0183579
PloS one 2017;12(8):e0183579
Evaluation of Antioxidant Intakes in Relation to Inflammatory Markers Expression Within the Normal Breast Tissue of Breast Cancer Patients.
Larouche D, Hanna M, Chang SL, Jacob S, Têtu B, Diorio C
Integrative cancer therapies 2017 Dec;16(4):485-495
Integrative cancer therapies 2017 Dec;16(4):485-495
Increased COX-2 expression in epithelial and stromal cells of high mammographic density tissues and in a xenograft model of mammographic density.
Chew GL, Huo CW, Huang D, Hill P, Cawson J, Frazer H, Hopper JL, Haviv I, Henderson MA, Britt K, Thompson EW
Breast cancer research and treatment 2015 Aug;153(1):89-99
Breast cancer research and treatment 2015 Aug;153(1):89-99
COX-2 overexpression and -8473 T/C polymorphism in 3' UTR in non-small cell lung cancer.
Bhat IA, Rasool R, Qasim I, Masoodi KZ, Paul SA, Bhat BA, Ganaie FA, Aziz SA, Shah ZA
Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2014 Nov;35(11):11209-18
Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2014 Nov;35(11):11209-18
Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson's disease.
Tapias V, Cannon JR, Greenamyre JT
Neurobiology of aging 2014 May;35(5):1162-76
Neurobiology of aging 2014 May;35(5):1162-76
Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-κB and HIF-1α signaling pathways.
Lei Q, Qiang F, Chao D, Di W, Guoqian Z, Bo Y, Lina Y
International journal of molecular medicine 2014 Dec;34(6):1629-39
International journal of molecular medicine 2014 Dec;34(6):1629-39
A quantitative proteomic analysis uncovers the relevance of CUL3 in bladder cancer aggressiveness.
Grau L, Luque-Garcia JL, González-Peramato P, Theodorescu D, Palou J, Fernandez-Gomez JM, Sánchez-Carbayo M
PloS one 2013;8(1):e53328
PloS one 2013;8(1):e53328
GRSF1 regulates RNA processing in mitochondrial RNA granules.
Jourdain AA, Koppen M, Wydro M, Rodley CD, Lightowlers RN, Chrzanowska-Lightowlers ZM, Martinou JC
Cell metabolism 2013 Mar 5;17(3):399-410
Cell metabolism 2013 Mar 5;17(3):399-410
Roles of E-cadherin and cyclooxygenase enzymes in predicting different survival patterns of optimally cytoreduced serous ovarian cancer patients.
Taşkin S, Dünder I, Erol E, Taşkin EA, Kiremitçi S, Öztuna D, Sertçelik A
Asian Pacific journal of cancer prevention : APJCP 2012;13(11):5715-9
Asian Pacific journal of cancer prevention : APJCP 2012;13(11):5715-9
Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure.
Lundström SL, Levänen B, Nording M, Klepczynska-Nyström A, Sköld M, Haeggström JZ, Grunewald J, Svartengren M, Hammock BD, Larsson BM, Eklund A, Wheelock ÅM, Wheelock CE
PloS one 2011;6(8):e23864
PloS one 2011;6(8):e23864
Cyclooxygenase-2 up-regulates vascular endothelial growth factor via a protein kinase C pathway in non-small cell lung cancer.
Luo H, Chen Z, Jin H, Zhuang M, Wang T, Su C, Lei Y, Zou J, Zhong B
Journal of experimental & clinical cancer research : CR 2011 Jan 10;30(1):6
Journal of experimental & clinical cancer research : CR 2011 Jan 10;30(1):6
Enhancement of osteoclastogenic activity in osteolytic prostate cancer cells by physical contact with osteoblasts.
Shiirevnyamba A, Takahashi T, Shan H, Ogawa H, Yano S, Kanayama H, Izumi K, Uehara H
British journal of cancer 2011 Feb 1;104(3):505-13
British journal of cancer 2011 Feb 1;104(3):505-13
Dissecting spatio-temporal protein networks driving human heart development and related disorders.
Lage K, Møllgård K, Greenway S, Wakimoto H, Gorham JM, Workman CT, Bendsen E, Hansen NT, Rigina O, Roque FS, Wiese C, Christoffels VM, Roberts AE, Smoot LB, Pu WT, Donahoe PK, Tommerup N, Brunak S, Seidman CE, Seidman JG, Larsen LA
Molecular systems biology 2010 Jun 22;6:381
Molecular systems biology 2010 Jun 22;6:381
Expression of cyclooxygenase-2 in choroidal neovascular membranes from age-related macular degeneration patients.
Maloney SC, Fernandes BF, Castiglione E, Antecka E, Martins C, Marshall JC, Di Cesare S, Logan P, Burnier MN Jr
Retina (Philadelphia, Pa.) 2009 Feb;29(2):176-80
Retina (Philadelphia, Pa.) 2009 Feb;29(2):176-80
Prognostic significance of cyclooxygenase-2 overexpression in glottic cancer.
Sackett MK, Bairati I, Meyer F, Jobin E, Lussier S, Fortin A, Gélinas M, Nabid A, Brochet F, Têtu B
Clinical cancer research : an official journal of the American Association for Cancer Research 2008 Jan 1;14(1):67-73
Clinical cancer research : an official journal of the American Association for Cancer Research 2008 Jan 1;14(1):67-73
The use of a cyclooxygenase-2 inhibitor (Nepafenac) in an ocular and metastatic animal model of uveal melanoma.
Marshall JC, Fernandes BF, Di Cesare S, Maloney SC, Logan PT, Antecka E, Burnier MN Jr
Carcinogenesis 2007 Sep;28(9):2053-8
Carcinogenesis 2007 Sep;28(9):2053-8
Expression of cyclo-oxygenase-2 in macrophages associated with cutaneous melanoma at different stages of progression.
Bianchini F, Massi D, Marconi C, Franchi A, Baroni G, Santucci M, Mannini A, Mugnai G, Calorini L
Prostaglandins & other lipid mediators 2007 Jun;83(4):320-8
Prostaglandins & other lipid mediators 2007 Jun;83(4):320-8
Cyclooxygenase-2 (COX-2) overexpression in meningiomas: real time PCR and immunohistochemistry.
Buccoliero AM, Castiglione F, Rossi Degl'Innocenti D, Arganini L, Taddei A, Ammannati F, Mennonna P, Taddei GL
Applied immunohistochemistry & molecular morphology : AIMM 2007 Jun;15(2):187-92
Applied immunohistochemistry & molecular morphology : AIMM 2007 Jun;15(2):187-92
Expression of protease-activated receptor-1 and -2 in orofacial granulomatosis.
Ketabchi S, Massi D, Ficarra G, Rubino I, Franchi A, Paglierani M, Simoni A, Capodiferro S, Favia G, Maiorano E, Tarantini F, Cirino G, Santucci M
Oral diseases 2007 Jul;13(4):419-25
Oral diseases 2007 Jul;13(4):419-25
The expression of cyclooxygenase 2 in retinoblastoma: primary enucleated eyes and enucleation after conservative treatment.
Souza Filho JP, Martins MC, Correa ZM, Odashiro AN, Antecka E, Coutinho AB, Macedo CR, Vianna RN, Burnier MN Jr
American journal of ophthalmology 2006 Oct;142(4):625-31
American journal of ophthalmology 2006 Oct;142(4):625-31
Pulmonary cryptosporidiosis: role of COX2 and NF-kB.
Asaad NY, Sadek GS
APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 2006 Oct;114(10):682-9
APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 2006 Oct;114(10):682-9
Cyclooxygenase-2 (COX-2) overexpression in childhood brain tumors.
Bodey B, Siegel SE, Kaiser HE
In vivo (Athens, Greece) 2006 Jul-Aug;20(4):519-25
In vivo (Athens, Greece) 2006 Jul-Aug;20(4):519-25
Cyclooxygenase-2 and inducible nitric oxide synthase expression in thyroid neoplasms and their clinicopathological correlation.
Kim KH, Kim SH, Kim SH, Back JH, Park MJ, Kim JM
Journal of Korean medical science 2006 Dec;21(6):1064-9
Journal of Korean medical science 2006 Dec;21(6):1064-9
Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid.
Birkenkamp-Demtroder K, Olesen SH, Sørensen FB, Laurberg S, Laiho P, Aaltonen LA, Orntoft TF
Gut 2005 Mar;54(3):374-84
Gut 2005 Mar;54(3):374-84
Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo.
Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, Sveinbjörnsson B, Kogner P
Cancer research 2004 Oct 15;64(20):7210-5
Cancer research 2004 Oct 15;64(20):7210-5
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
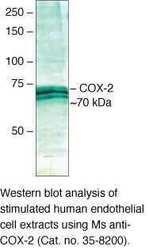
- Experimental details
- Western blot analysis of stimulated human endothelial cell extracts using Ms anti-COX-2 (Product # 35-8200).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western Blot was performed using Anti-COX2 Monoclonal Antibody (COX 229) (Product # 35-8200) and a 69 kDa band corresponding to Prostaglandin G/H synthase 2 was observed across the cell lines tested. Whole cell extracts (30 µg lysate) of U-937 (Lane 1), HUVEC (Lane 2), HUVEC treated with 1 ng/mL of IL-1ß for 24 hours (Lane 3) were electrophoresed using NuPAGE™ 4-12% Bis-Tris Protein Gel (Product # NP0321BOX). Resolved proteins were then transferred onto a nitrocellulose membrane (Product # LC2001) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody at a concentration of 1 µg/mL and detected by chemiluminescence with Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000 dilution using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
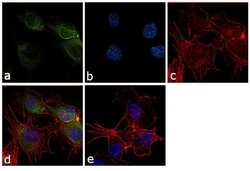
- Experimental details
- Immunofluorescence analysis of COX-2 was performed using 70% confluent log phase HUVEC cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with COX2 (COX 229) Mouse Monoclonal Antibody (Product # 35-8200) at 2µg/mLin 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Fluorescent immunocytochemistry was performed on cytospun BAL-cells using monoclonal antibodies against COX-1, COX-2, 15-LOX-1 and PPARgamma coupled with secondary antibodies labeled with fluorescent dyes Alexa488 (green) and Cy5 (red). Nuclear counter-staining was performed using DAPI (blue). Representative micrographs from a healthy and an asthmatic individual are shown for both the control air (A) and subway air (B) exposures. Semi-quantitative evaluation of staining intensities was performed using ImageJ software [24] .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
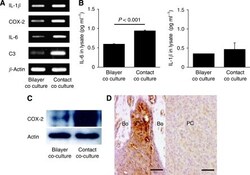
- Experimental details
- Figure 3 Analysis of IL-1 beta , COX-2, IL-6 and C3 expression in PC3 cells cocultured with hFOB1.19 cells. ( A ) The mRNA expression of the four osteoclastogenesis-related genes, IL-1 beta , COX-2, IL-6 and C3, in PC-3 cells following coculture under contact and bilayer conditions, was analysed using RT-PCR. Consistent with the cDNA microarray results, these genes were upregulated in contact cocultured PC-3 cells compared with bilayer cocultured cells. The primers used for RT-PCR are shown in Table 1 . ( B ) Measurement of IL-6 and IL-1 beta levels in PC-3 cells using ELISA. The level of IL-6 was significantly higher ( P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 GRSF1 Is Required for Mitochondrial Gene Expression (A) Left panel, immunoblot analysis of 143B cells treated with control (Ctrl) or GRSF1 RNAi1. Right panel, immunoblot analysis of 143B cells infected with lentiviruses carrying an empty vector (pLKO) or a shRNA (RNAi2) against GRSF1. COX1 and COX2 are mitochondrially encoded proteins. SDHB and Tom20 are nuclear-encoded mitochondrial proteins. Actin is a nuclear-encoded cytosolic protein. (B) Acidification of the culture medium in GRSF1 RNAi1-treated 143B cells. The number of cells was the same in both conditions. Similar results were obtained in GRSF1 RNAi2-infected cells (data not shown). (C) NanoString analysis of 11 mitochondrially encoded ORFs in control (Ctrl) or GRSF1 RNAi1-treated 143B cells. RNA14 corresponds to bicistronic MTATP8 - MTATP6 RNA. Data are shown as mean +- SEM (n = 3). (D) NanoString analysis of GRSF1 in control (Ctrl) or GRSF1 RNAi1-treated 143B cells. Data are shown as mean +- SEM (n = 3). (E) NanoString analysis of 11 nuclear-encoded proteins in control (Ctrl) or GRSF1 RNAi1-treated 143B cells. Data are shown as mean +- SEM (n = 3). (F) Northern blot analysis of 12S and 16S rRNA in control (Ctrl) or GRSF1 RNAi1-treated cells. TUB, tubulin. (G) Phosphorimager quantification of (E). Data are shown as mean +- SEM (n = 3). (H) 35 S-labeling of mitochondrial translation of 143B cells infected with lentiviruses carrying an empty vector (pLKO) or a shRNA (RNAi2) against GRSF1. (I) Phosphorimager qu
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
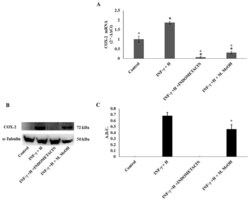
- Experimental details
- Figure 3 Cyclooxygenase-2 (COX-2) mRNA expression ( A ) and protein production ( B, and C ). COX-2 mRNA expression was determined by RT-PCR ( A ), and COX-2 protein production was determined using Western blot ( B : representative immunoblot; C: protein expression calculated as Arbitrary Densitometric Units; A.D.U.) in the NCTC 2544 72 h after the addition of M. MeOH (50 ug/mL) with INF-gamma + H. * Significantly different than control; deg significantly different than INF-gamma/H-treated samples ( p < 0.05).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 1 Immunohistochemical staining demonstrates the intensity of COX-2 expression in the cytoplasm of follicular carcinoma cases. ( A ) score 0; ( B ) score 1; ( C ) score 2; ( D ) score 3 (x400).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 3 Loss of N-AS generation by Abeta reduces COX2 acetylation and SPMs production in neurons and microglia, except astrocyte. a The primary culture of neuron, microglia, and astrocyte was prepared from C57BL/6 mice, and N-AS were detected by LC-MS/MS in these cells. Representative chromatograms of N-AS and quantification in neuron, microglia, and astrocyte treated 10 muM Abeta or not ( n = 6 per group). b Western blotting for ac-S565 and total COX2 in neuron, microglia, and astrocyte treated 10 muM Abeta or not ( n = 6 per group). c Immunofluorescence images and quantification of neuron (NeuN, red), microglia (Iba1, red), or astrocyte (GFAP, red) with ac-S565 (green) and COX2 (blue) ( n = 6 per group, scale bars, 50 mum). d SphK activity in neuron and microglia treated 10 muM Abeta or not ( n = 6 per group). e Analysis of acetyl-CoA in neuron and microglia treated 10 muM Abeta or not using assay kit ( n = 6 per group). f Detection of sphingosine in neuron and microglia treated 10 muM Abeta or not using UPLC ( n = 6 per group). g Quantification of N-AS by LC-MS/MS in neuron and microglia treated 10 muM Abeta or not in presence of acetyl-CoA, sphingosine, or SphK1 each ( n = 6 per group). h Western blotting for ac-S565 in microglia and neuron treated 10 muM Abeta or not in presence of N-AS, acetyl-CoA, sphingosine, or SphK1 each ( n = 6 per group). i Quantification of 15R-LXA 4 , RvE1, and RvD1 using systematic LC-MS/MS in microglia and neuron treated 10 muM Abeta or not in
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
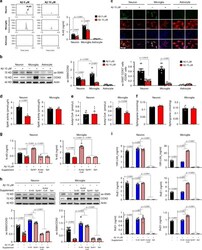
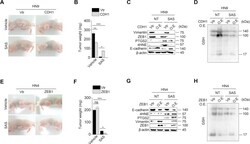
- Fig. 2 Inhibition of CDH1 increases the susceptibility of HNC cells to ferroptosis inducers. ( A ) Immunoblotting of vimentin, ZEB1, GPX4, Nrf2, and E-cadherin with or without CDH1 silencing in HN4 cancer cells. Ctr, control; scr, scrambled. ( B - C ) Cell death was measured using PI staining, in HN4 with or without CDH1 gene silencing and exposure to 1 muM RSL3 or 0.5 mM sulfasalazine (SAS) treatment for 72 h. Original magnification, x 200. Scale bar, 50 mum. ( D ) Labile iron pool (LIP) was measured by calcein-AM (8 mug/ml) after 1 muM RSL3 or 0.5 mM SAS; all data were quantified by the Image J software. ( E , F ) Lipid and cytosolic ROS were measured using flow cytometry with staining BODIPY C11 and DCFDA, respectively, after 1 muM RSL3 or 0.5 mM SAS treatment, or cyst(e)ine deprivation for 24 h. ( G ) Immunoblotting of vimentin, ZEB1, GPX4, Nrf2, and E-cadherin with or without transfection of CDH1 overexpression or control vector. ( H ) Immunoblotting of 4-HNE, PTGS2, and CDH1 in HN6 cells with or without CDH1 overexpression. ( I - L ) PI staining, LIP assay, lipid peroxidation (BODIPY C11), and total ROS (DCFDA) measurements in HN6 cells transfected with or without CDH-1 overexpression and then exposure to 1 muM RSL3 or 0.5 mM SAS, or cyst(e)ine deprivation. Scale bar 50 mum. The error bars represent standard errors from three replicates. ** P < 0.01, *** P < 0.001 between the control and CDH1 overexpression. ( M - N ) Tumor volume, glutathione (GSH) content, and NAD/NAD
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 3 ZEB-1 regulates ferroptosis sensitivity. ( A ) Immunoblotting of E-cadherin, vimentin, GPX4, Nrf2, and ZEB1 in HN6 cells with or without ZEB1 silencing. ( B - E ) Cell death, labile iron pool (LIP), lipid peroxidation, and total ROS assays were measured in HN6 cancer cells with or without ZEB1 gene silencing after 1 muM RSL3 or 0.5 mM SAS or cyst(e)ine deprivation. ( F ) Immunoblotting of E-cadherin, ZEB1, vimentin, Nrf2 and GPX4 in HN4 cells with or without ZEB1 overexpression. ( G ) Immunoblotting of 4-HNE, PTGS2, and ZEB1 in HN4 cells with or without ZEB1 overexpression. ( H - L ) Cell death, LIP, lipid peroxidation, and total ROS assays were also measured in HN4 cancer cells with or without ZEB1 overexpression after exposure to 1 muM RSL3 and 0.5 mM SAS. Original magnification, x 200. Scale bar, 50 mum. The error bars represent standard errors from three technical replicates. ** P < 0.01, *** P < 0.001 between the control and ZEB1 overexpression. ( M - N ) GSH content and NAD/NADH ratio were measured in HN3 and HN4 cells with ZEB1 overexpression or control vector. ( O - Q ) Tumor volume, GSH content, and NAD/NADH ratio were assessed in HN4 tumors with or without ZEB overexpression that were transplanted and grown in nude mice. * P < 0.05, ** P < 0.01, *** P < 0.001 between the vector control and ZEB1 overexpression or between the vehicle control and SAS treatment groups. Fig. 3
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. S4 CDH1 or ZEB1 regulation modulates the susceptibility to sulfasalazine in vivo . ( A - D ) Representative images, weights, and immunoblotting of tumors grown in mice with HN9 cells transfected with CDH1 overexpression or control vector. The mice were subjected to vehicle or SAS (250 mg/kg daily per intraperitoneal route) for 21 days. Immunoblotting was performed from tumors extracted at 21 days with the primary antibodies of vimentin, ZEB1, PTGS2, 4HNE, E-cadherin, GSH, and beta-actin. ( E - H ) Representative images, weights, and immunoblotting of tumors harvested in mice HN4 cells with or without ZEB1 overexpression or control vector. The mice were also subjected to SAS or vehicle for 21 days. * P < 0.05, * P < 0.01, *** P < 0.001 between the vector control and CDH1 or ZEB1 overexpression or between vehicle or SAS treatment. Fig. S4
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2 Palmitate and IL-26 synergistically induced the production of inflammatory factors by HACs. Primary HACs were treated with 0.25 mM palmitate alone, 100 ng/mL of IL-26, or a combination of both for 24 h. Total protein was extracted, and inflammatory or extracellular matrix proteins (COX-2, IL-6, MMP-1, and COL-II) were quantified by Western blot analysis. Data are presented as the mean +- SD. Significant differences were detected between the control and palmitate and/or IL-26 groups ( n = 3).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
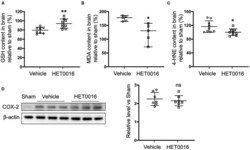
- Figure 5 HET0016 decreases lipid peroxidation level after ICH mice. Glutathione (GSH) (A) , malondialdehyde (MDA) (B) , and 4-hydroxy-2-nonenal (4-HNE) (C) content in brain 3 days after ICH. HET0016 reversed the ICH-induced reduction in GSH and increases in MDA and 4-HNE. n = 9 (A,C) or 5 (B) per group. (D) Brain tissue surrounding the hematoma was harvested 3 days after ICH, and total protein was extracted for western blotting. After ICH, cyclooxygenase-2 (COX-2) levels increased to a similar extent in the vehicle and HET0016 groups relative to that in the Sham group. n = 7/group. * P < 0.05, ** P < 0.01 vs. Vehicle group. Mann-Whitney U -test (B) or unpaired t -test (A,C,D) was applied. Data are expressed as median with confidence interval (B) or mean +- SD (A,C,D) .
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA